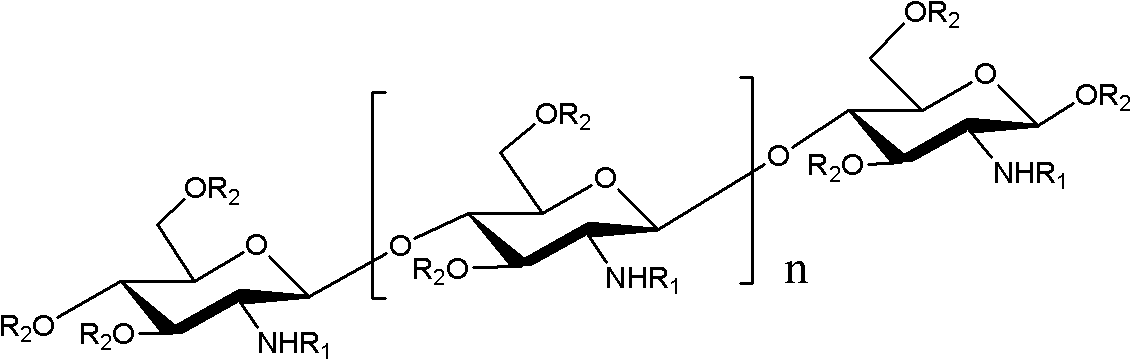
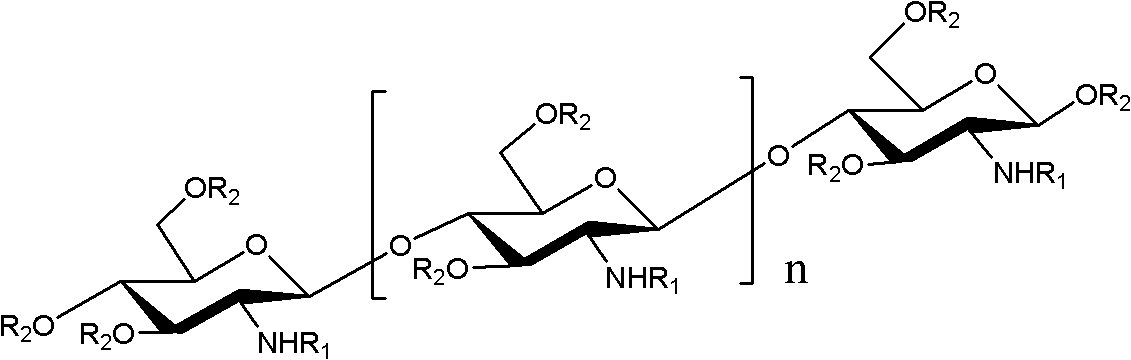
O-unsaturated fatty acid acylated chitosan oligosaccharides as well as preparation and application thereof
A technique for fatty acid acylation of chitooligosaccharides and unsaturated fatty acids, which is applied in the field of O-unsaturated fatty acid acylation of chitooligosaccharides and its preparation, and can solve problems such as insufficient activity, insufficient stability of unsaturated fatty acids, troublesome use and storage, etc. , to achieve the effect of high selectivity, clear structure and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of O-linoleic acid acylated chitooligosaccharide
[0031] Take 1.62g of chitosan oligosaccharides with a degree of polymerization of 2-8 and dissolve in 30mL of N,N-dimethylformamide, add 1.63g of p-methoxybenzaldehyde, stir at room temperature for 4 hours, and add 5 to the reaction solution. A double volume of ethanol produces a large amount of light yellow precipitate, which is suction filtered. The filter cake is washed 3 times with anhydrous acetone and dried under vacuum to obtain 2.5 g of light yellow powder, which is an amino schiff base protected chitosan oligosaccharide.
[0032] Take 2.8g amino-protected chitosan oligosaccharide and dissolve it in N,N-dimethylformamide and N,N-dimethylformamide; then add 0.1g dimethylaminopyridine; add 3.0g linoleic acid N , N-dimethylformamide, react the above solution at 60°C for 6 hours, add 4 times the volume of ethanol to the reaction solution to produce a large amount of light yellow precipitate, suctio...
Embodiment 2
[0036] Example 2: Preparation of O-linolenic acid acylated chitosan oligosaccharide
[0037] Take 1.62g of chitosan with a degree of polymerization of 2-15 and dissolve in 30mL of N,N-dimethylformamide, add 1.63g of benzaldehyde, stir at room temperature for 4 hours, and add 5 times its volume of ethanol to the reaction solution , A large amount of light yellow precipitate is produced, filtered by suction, the filter cake is washed 3 times with anhydrous acetone, and dried in vacuum to obtain 2.5 g of light yellow powder, which is an amino schiff base protected chitosan oligosaccharide.
[0038] Dissolve 2.8g amino-protected chitosan oligosaccharide in N,N-dimethylformamide and N,N-dimethylformamide; add 0.1g dimethylaminopyridine; add 4.1g linolenic acid N, N-Dimethylformamide, react the above solution at 60°C for 8 hours, add 4 times its volume of ethanol to the reaction solution to produce a large amount of light yellow precipitate, filter with suction, and wash the filter cake ...
Embodiment 3
[0042] Example 3: Myristic acid, palmitoleic acid, oleic acid, stearidonic acid, eicosapentaenoic acid, docosahexaenoic acid, octadecadienoic acid, octadecatriene Preparation of O-acylated chitosan oligosaccharides such as acid and eicosatetraenoic acid
[0043] According to the method of Examples 1 and 2, the unsaturated fatty acid raw materials are selected from myristic acid, palmitoleic acid, oleic acid, stearidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and ten Octadienoic acid, octadecadienoic acid, and eicosatetraenoic acid can be prepared to obtain O-myristoleic acid acylated chitooligosaccharides, O-palmitoleic acid acylated chitosan oligosaccharides, and O-oleic acid Acylated chito-oligosaccharides, O-octadecosatrienoic acid acylated chito-oligosaccharides, O-eicosapentaenoic acid acylated chito-oligosaccharides, O-docosahexaenoic acid acylated chito-oligosaccharides, O -Octadecadienoic acid acylated chitosan oligosaccharide, O-octadecadienoic acid acylated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com