Malignant malaria vaccine and preparation method thereof
A technology for microbial strains and silkworms, which is applied in the field of biomedicine, can solve the problems of high cost, low expression, and inability to produce neutralizing antibodies, and achieves the effects of high yield, high application value and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Construction of recombinant transfer plasmid pFastBacI-gp64-AMA1
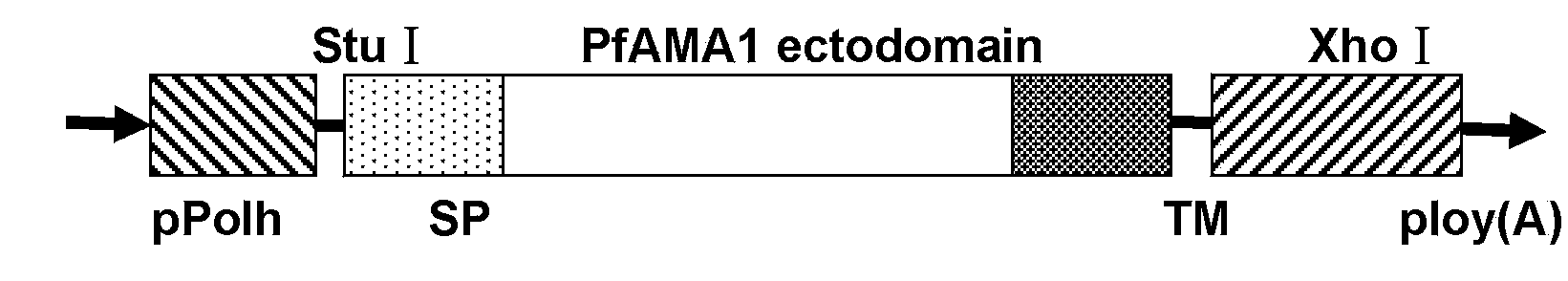
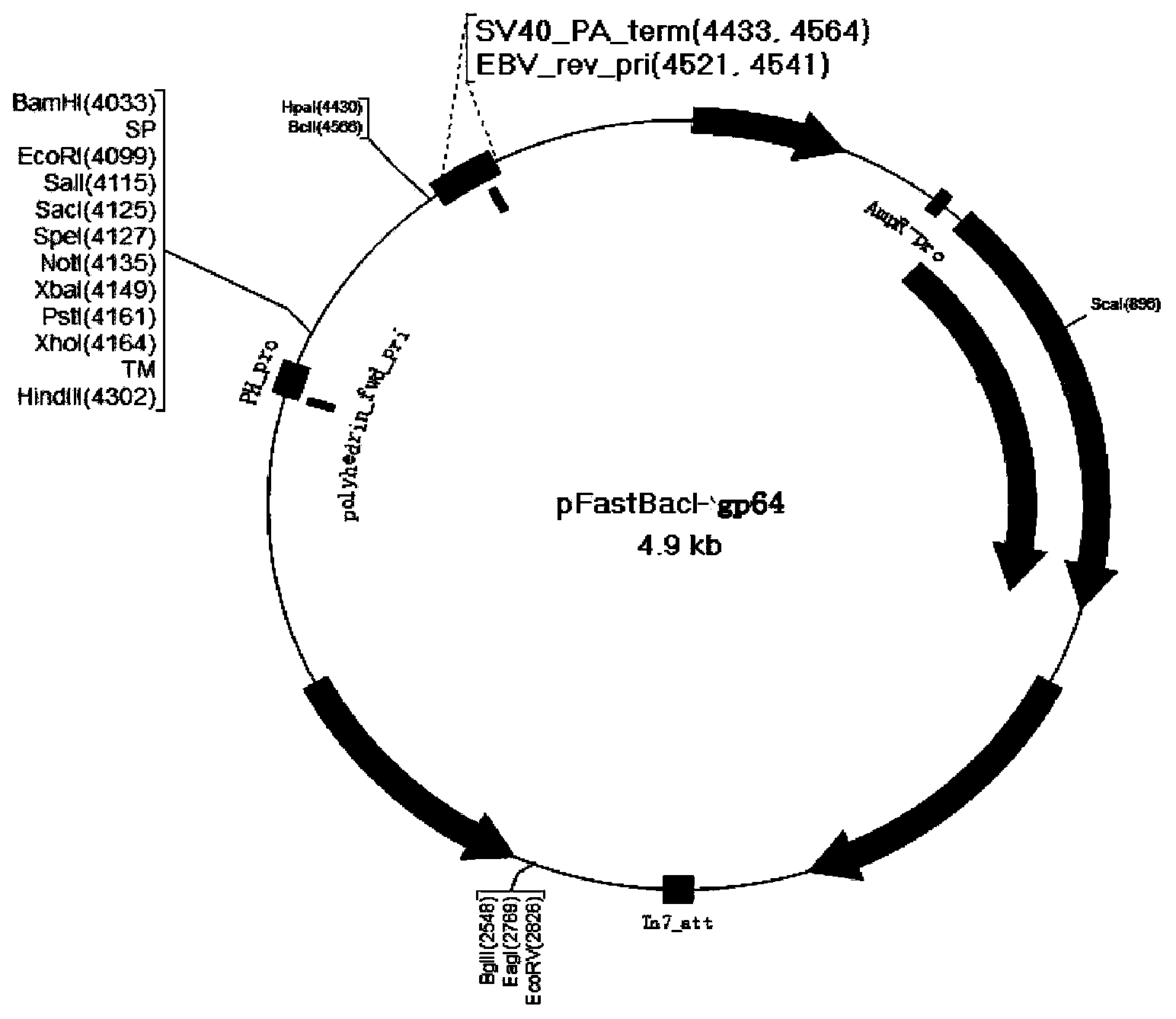
[0029] Using the baculovirus gp64 sequence as a template, PCR amplified the signal peptide (SP) sequence and transmembrane sequence (TM) of gp64 with primers P1, P2, P3, and P4, and the PCR product passed Bam H I / EcoR I and Xho I / Hind Ⅲ Double enzyme digestion inserts the upstream and downstream ends of the multiple cloning site of pFastBacI vector to construct the display vector pFstBacI-gp64 (vector structure is as figure 1 Shown). Then, using the cDNA of the standard strain of Plasmodium falciparum 3D7 as a template, PCR was performed with primers P5 and P6 to amplify the AMA1 ectodomain of the apical membrane antigen of Plasmodium falciparum. The PCR product passed Stu I and Xho I double enzyme digestion into the surface display vector pFastBacI-gp64 to construct a recombinant transfer vector pFastBacI-gp64-AMA1. The recombinant transfer vector includes polyhedron promoter (pPol...
Embodiment 2
[0060] Example 2: Obtaining the Bombyx mori recombinant baculovirus Bmgp64AMA1
[0061] Identify the successfully recombined recombinant transfer plasmid pFastBacI-gp64-AMA1 to transform E. coli DH10Bac competent cells (purchased from Invitrogen) on an LB culture plate containing kanamycin, gentamicin, tetracycline, X-gal and IPTG ( (Purchased from Shanghai Shenggong Biological Company) for blue-white spot screening. After 48 hours of dark culture, the white spots were picked. After 24 hours of culture, the recombinant baculovirus genome was extracted with isopropanol, and the M13 universal primers were used for PCR identification. The successfully identified recombinant virus genome was transfected into Bombyx mori BmN cells (purchased from Invitrogen) by liposome-mediated method (refer to the instructions of Invitrogen’s liposome transfection reagent Cellfectin Ⅱ Reagent). After the onset of disease (microscopic observation), a first-generation virus was obtained. The suspensio...
Embodiment 3
[0062] Example 3: Expression of AMA1 fusion protein in 5th instar larvae and pupae of silkworm
[0063] The silkworm recombinant baculovirus Bmgp64AMA1 was infected with 10MOI virus to BmN cells for viral amplification, and the 5th-instar larvae or silkworm pupae of silkworm (purchased from Zhejiang Zhongqi Biopharmaceutical Co., Ltd.) were inoculated with 1×10 PFU per acupuncture method. After 5-7 days of infection, the larvae or pupae were collected and centrifuged at a high speed (12000rpm, 30min). The supernatant was taken to detect the expression of recombinant protein. Add an equal volume of 2× protein loading buffer (100Mm Tris HCl, 4% SDS, 0.15% bromophenol blue, 10% glycerol) to the supernatant after high-speed centrifugation, heat it at 100°C for 10 min, and take 20 μl for SDS-PAGE analysis. The results show that the silkworm recombinant baculovirus has expressed the AMA1 fusion protein, and the protein sequencing results show that its amino acid sequence is as shown in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com