Method for synthesizing novel reactive ultraviolet absorbent and application thereof
A UV absorber, reactive technology, used in organic chemistry, textiles and papermaking, fiber processing, etc., can solve the problems of lack of affinity, not easy to fiber, easy to diffuse, etc., and achieve good thermal stability, good soaping resistance, The effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
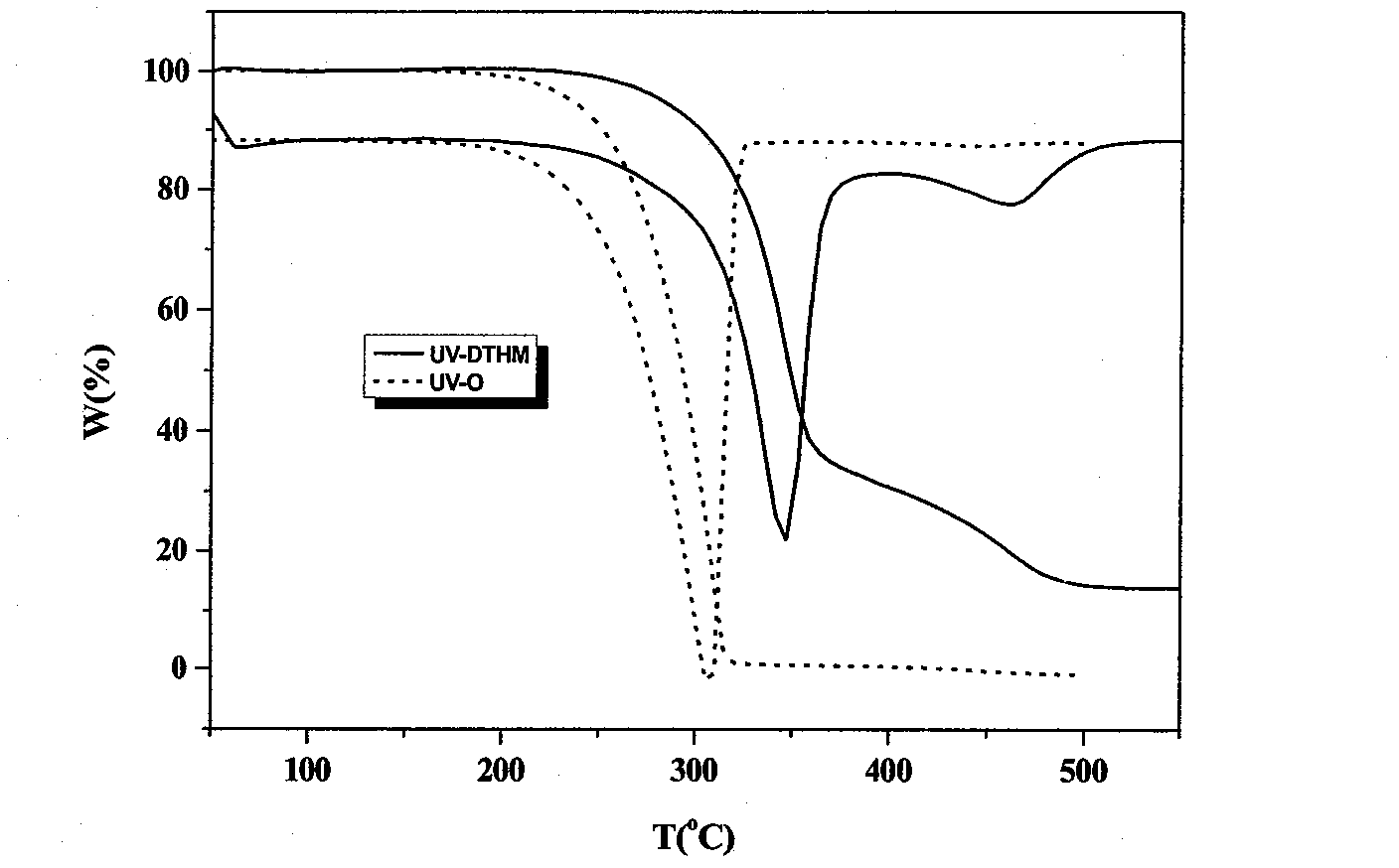
[0046] Embodiment 1: the synthesis of UV-DTHM
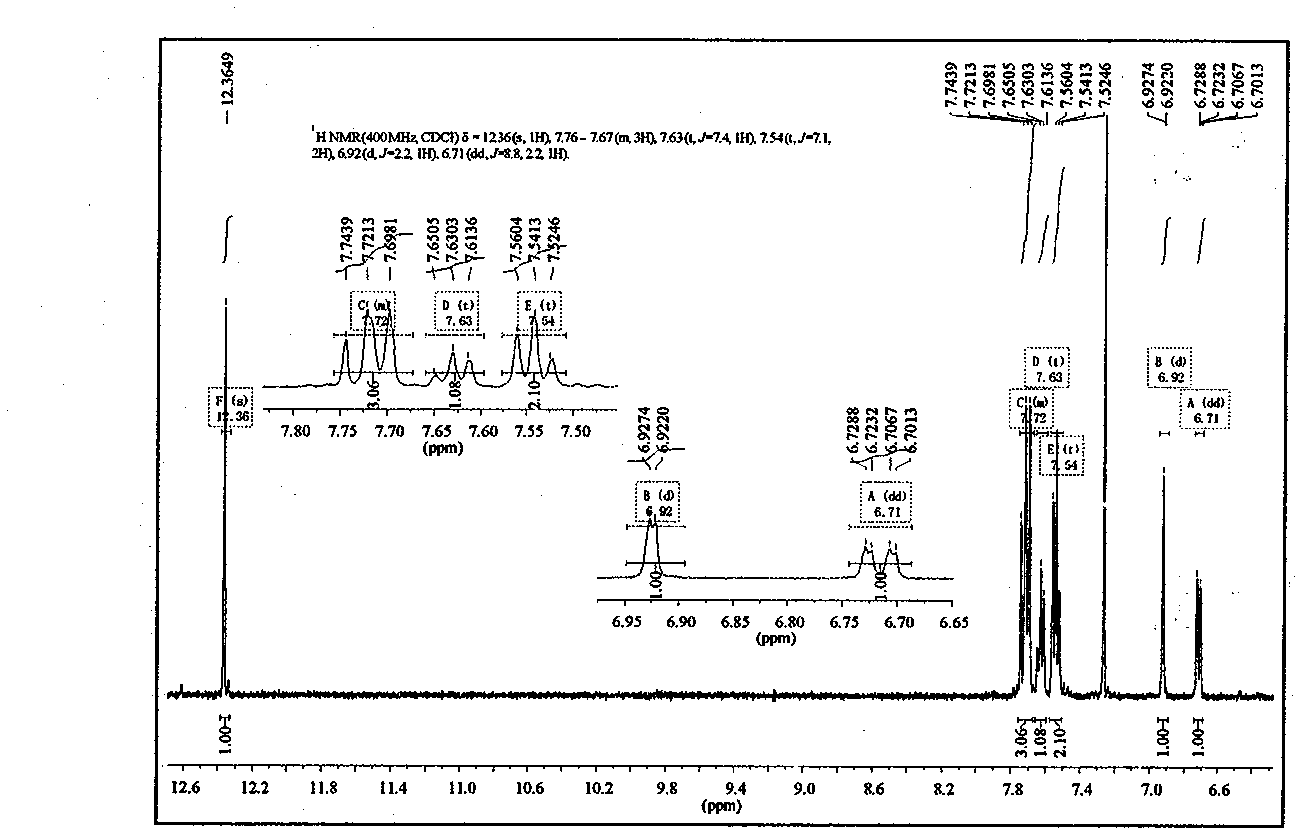
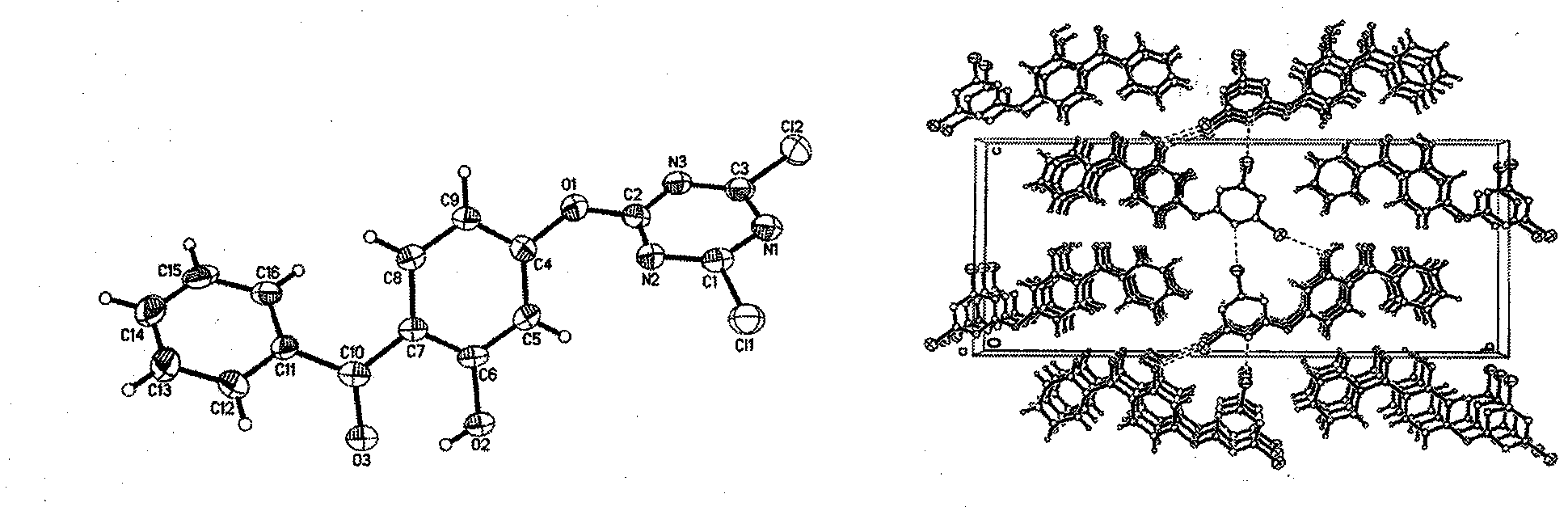
[0047]Add 2.583g (0.014mol) of cyanuric chloride and 20mL of acetone into a three-necked flask, stir and swell for 10min under ice bath conditions, then dissolve 1.4995g (0.007mol) of UV-O and 0.42g (0.0105mol) of NaOH in 15mL of acetone and 50mL of distilled water, and after the two solutions were evenly mixed, slowly added to a three-necked flask, and reacted at 0°C for 5h to obtain a light yellow powder, which was followed by TLC. After the reaction, suction filtration, The filter cake was washed with distilled water and ethanol, respectively, and repeated twice to obtain a dry crude product. Separation and purification by column chromatography (eluent: ethyl acetate:petroleum ether=1:7) gave the product as light yellow powdery solid. Yield 82.5%. Through the recrystallization of dichloromethane, a UV-DTHM single crystal was obtained, and the crystal structure was determined. For the crystal structure, see image 3 , the cr...
Embodiment 2
[0053] Embodiment 2: UV-DTHM is applied to finishing cotton fabric
[0054] Dissolve 1% (owf) UV absorber in 2mL toluene, then add it to the aqueous solution containing 5% (owf) tetradecyltrimethylammonium chloride, stir evenly, and heat in a water bath to After 60°C, put the cotton fabric soaked in water in advance into the solution, the bath ratio is 1:20, and add 20g / L sodium bicarbonate at the same time, react for 30min, then raise the temperature to 85°C, add 10g / L Na 2 CO 3 , continue to react for 60 minutes, take out the fabric, wash it with water, and dry it at 100°C for 30 minutes to obtain the finished cotton fabric. Use a textile ultraviolet protection factor tester (Cary50) to measure the ultraviolet absorption effect, and the UPF value is 66.8±3. At the same time, the properties of the finished fabric were measured (see Table 4).
Embodiment 3~6
[0055] Embodiment 3~6: the washing resistance test of UV-DTHM finishing cotton fabric
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com