Preparation method of SSZ-13 loaded Cu-Fe catalyst for selectively catalyzing and eliminating NOx by ammonia
A catalyst, selective technology, applied in physical/chemical process catalysts, molecular sieve catalysts, chemical instruments and methods, etc., to achieve high hydrothermal stability, high stability, and stable activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
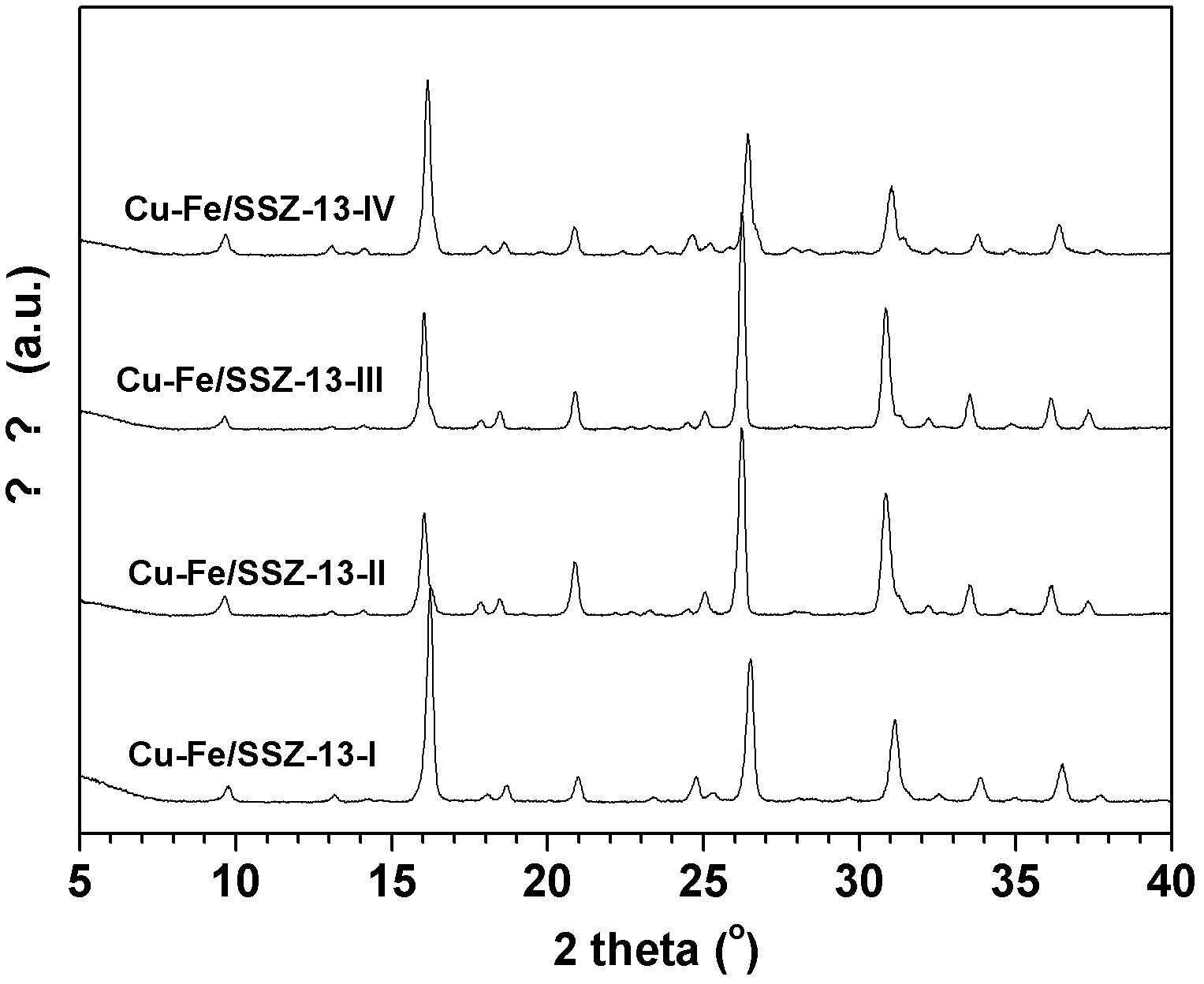
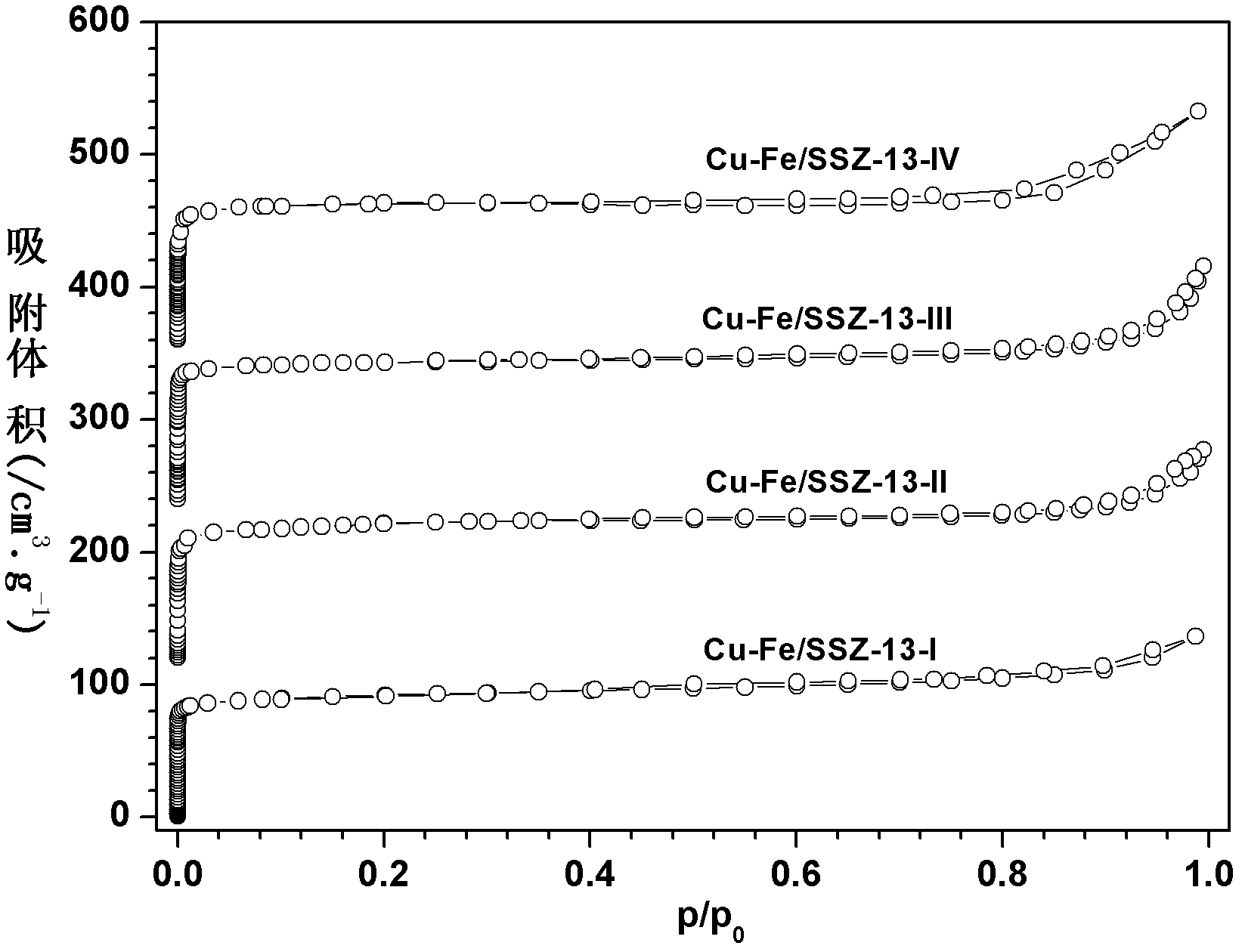
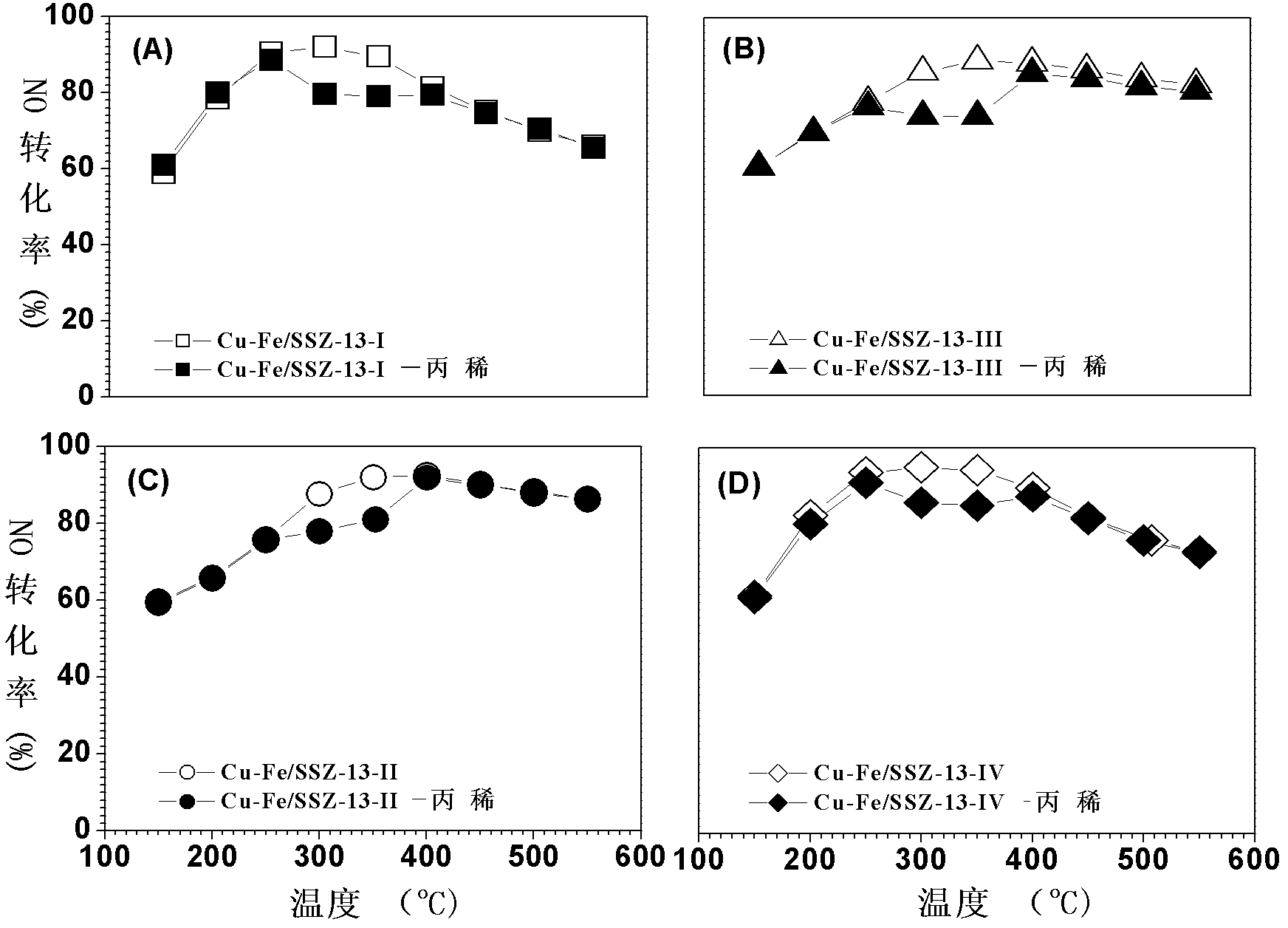
Image
Examples
Embodiment 1
[0024] (1) Preparation of SSZ-13 molecular sieve
[0025] Dissolve sodium silicate solution and N,N,N-trimethyl-1-adamantammonium iodide R (N,N,N-trimethyl-1-adamantammonium iodide) in water to make the first solution, room temperature Let stand for 4 days. al 2 (SO 4 ) 3 .16H 2 O and concentrated NaOH aqueous solution were dissolved in water to make the second solution, which was allowed to stand at room temperature for 4 days. Add the second solution to the first solution, stir until uniform, obtain a milk-colored solution, and finally obtain the sol composition (molar molecular weight ratio) as follows:
[0026] 61SiO 2 : 1.0Al 2 o 3 :38Na 2 O:9.75R:1238H 2 o
[0027] The above solution was left to stand in an airtight container for 10 days. Then the sol was added to a stainless steel reactor lined with polytetrafluoroethylene, heated to 120°C, and kept at a constant temperature for 5 days. The solid product was then filtered, washed and dried in air at 30°C. ...
Embodiment 2
[0034] (1) Preparation of SSZ-13 molecular sieve
[0035] Dissolve sodium silicate solution and N,N,N-trimethyl-1-adamantammonium iodide R (N,N,N-trimethyl-1-adamantammonium iodide) in water to make the first solution, room temperature Let stand for 5 days. al 2 (SO 4 ) 3 .16H 2 O and concentrated NaOH aqueous solution were dissolved in water to make the second solution, and left at room temperature for 5 days. Add the second solution to the first solution, stir until uniform, obtain a milk-colored solution, and finally obtain the sol composition (molar molecular weight ratio) as follows:
[0036] 30SiO 2 : 1.0Al 2 o 3 : 15Na 2 O: 4.88R: 960H 2 o
[0037] The above solution was left to stand in an airtight container for 12 days. Then the sol was added to a stainless steel reactor lined with polytetrafluoroethylene, heated to 140°C, and kept at a constant temperature for 7 days. The solid product was then filtered, washed and dried in air at 50°C. In order to remo...
Embodiment 3
[0044] (1) Preparation of SSZ-13 molecular sieve
[0045] Dissolve sodium silicate solution and N,N,N-trimethyl-1-adamantammonium iodide R (N,N,N-trimethyl-1-adamantammonium iodide) in water to make the first solution, room temperature Let stand for 6 days. al 2 (SO 4 ) 3 .16H 2 O and concentrated NaOH aqueous solution were dissolved in water to make the second solution, which was allowed to stand at room temperature for 6 days. Add the second solution to the first solution, stir until uniform, obtain a milk-colored solution, and finally obtain the sol composition (molar molecular weight ratio) as follows:
[0046] 31SiO 2 : 1.0Al 2 o 3 : 17Na 2O: 3.9R: 968H 2 o
[0047] The above solution was left to stand in an airtight container for 13 days. Then the sol was added to a stainless steel reactor lined with polytetrafluoroethylene, heated to 160°C, and kept at a constant temperature for 6 days. The solid product was then filtered, washed and dried in air at 70°C. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Total pore volume | aaaaa | aaaaa |
| Total pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com