Preparation method of human protein lysine methyltransferase SET9 fusion protein
A technology of lysine methyl and fusion protein is applied in the field of genetic engineering Escherichia coli fermentation to prepare recombinant human SET9 fusion protein, which can solve the problems of insolubility and difficulty in increasing active SET9 protein extraction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1. PCR amplification of SET9 gene fragment
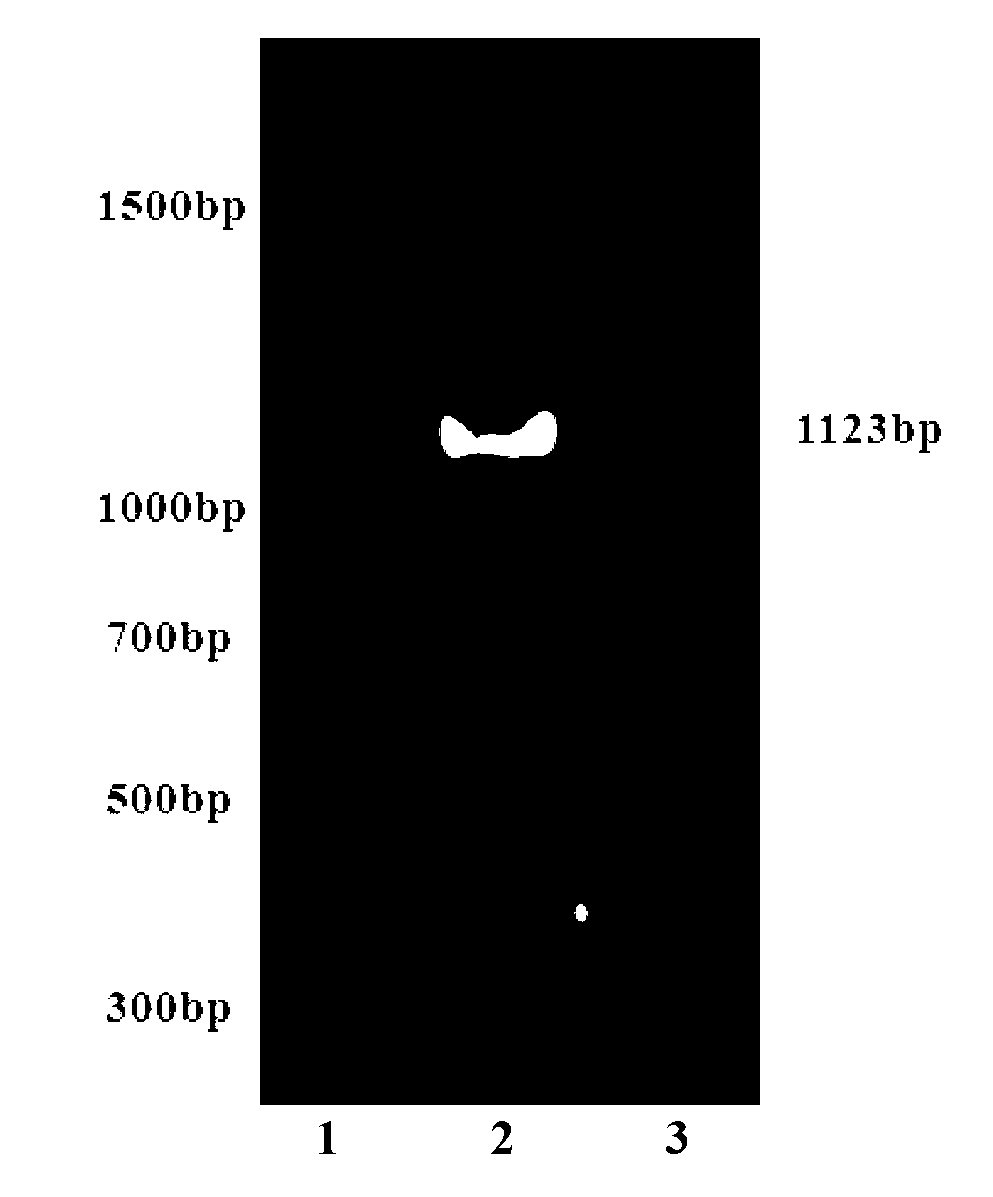
[0015] Using pCMV-SET9 (purchased from Clontech, USA) as a template, the SET9 gene fragment containing the SET domain was amplified by PCR. The cDNA sequence of SET9 was determined by NCBI GenBank (accession No, AF448510), EcoRI and Xho I were selected as restriction sites, and DNA Star software was used to design SET9 (EcoRI---Xho I) PCR primers and upstream primers (SET9-f) The sequence is: 5'-ACCGAATTCCATGGATAGCGACGAC-3', containing the EcoR I restriction site (G↓AATTC) and the initiation codon (ATG); the sequence of the downstream primer (SET9-r) is: 5'-CTCCTCGAGTCATTACTTTTGCTGGGTGGCCTG-3', Contains a double stop codon (TCATTA) and an XhoI restriction site (C↓TCGAG), located at the 268th to 1365th nucleotide of the complete gene sequence of SET9. Product: Length=1098+10+15=1123bp, GC%=50.0.
[0016] reaction system:
[0017] Reagent
Volume(μL)
10x PCR Buffer (Pfu)
5
Pfu (2.5u / μL, Strata...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com