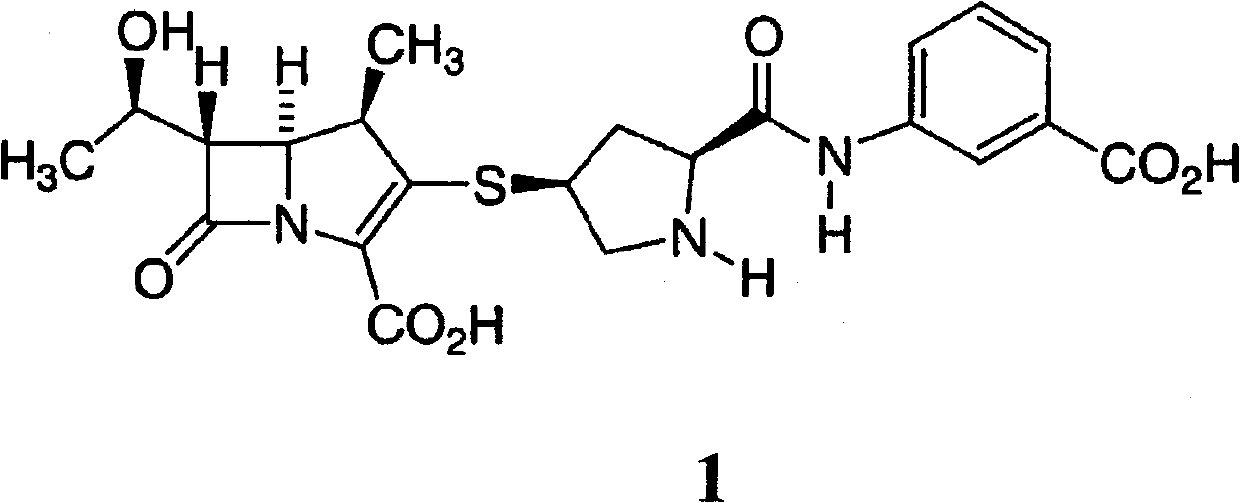
Preparation method of ertapenem and its sodium salt
A technology of ertapenem and sodium salt is applied in the field of preparation of carbapenem compound ertapenem and its sodium salt, and can solve the problems of difficult removal, excessive heavy metals, poor product purity and the like, and achieves improved product purity, Reduce the effect of product degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
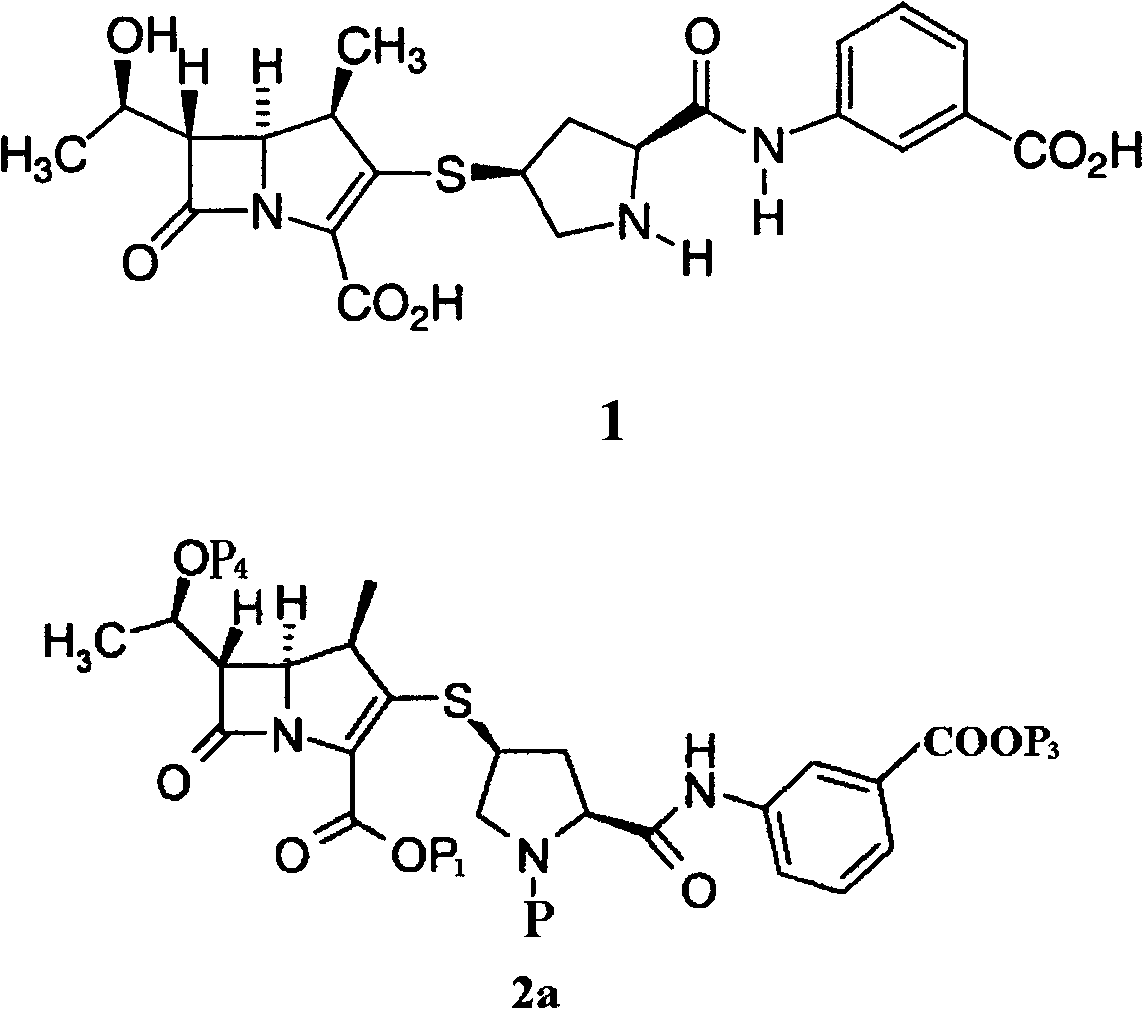
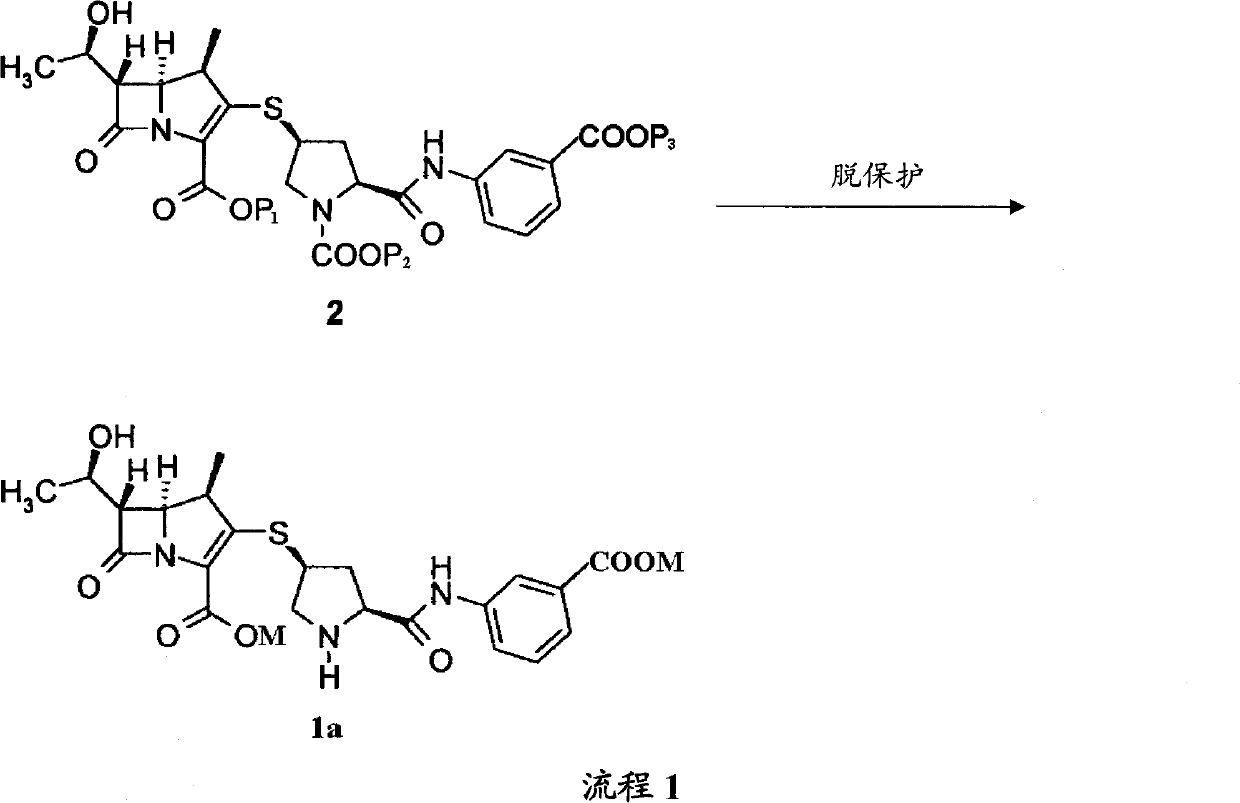
[0037] Preparation Example 1P 1 ,P 2 Both are PNB, P 3 ,P 4 Preparation of compound 2a for H
[0038] Compound 3(P 1 PNB) 36.0g was dissolved in 300ml DMF, compound 4 (P 2 PNB) 26.7g, 7.3g of diisopropylethylamine was slowly added dropwise at -35°C, and reacted under stirring. After completion of the reaction, the reaction solution was added to the acid aqueous solution of pH 2~6 to obtain 46.6g of compound 2a (P 1 ,P 2 Both are PNB, P 3 is H) solid.
[0039] P 1 ,P 2 For other carboxyl protecting groups (including P is HCl), P 3 The preparation of compound 2a which is H can refer to the method described in Preparation Example 1.
preparation example 2
[0040] Preparation Example 2P 1 ,P 2 Both are PNB, P 3 for Na + ,P 4 Preparation of compound 2a for H
[0041] Compound 3(P 1 is PNB) 100g and compound 4 (P 2 for PNB, P 3 Dissolve 73.8g of H) in DMF, add 60mL of DBU solution in DMF at -50°C, stir, after the reaction is complete, pour the reaction solution into ethyl acetate and buffer solution (175g potassium dihydrogen phosphate dissolved in water or hydrogen phosphate In the mixed solution of dipotassium buffer solution), adjust the pH value of the two-phase system to 4.5, separate the liquids, add the ethyl acetate solution of sodium isooctanoate (32g) to the organic phase, obtain a pasty substance, and slowly pour out the organic phase layer, the paste substance was dissolved in methanol, and isopropyl ether was added to the methanol solution to obtain 110g of compound 2a (P 1 ,P 2 Both are PNB, P 3 for Na + )solid.
[0042] P 1 ,P 2 For other carboxyl protecting groups (including P is HCl) and P 3 The prep...
preparation example 3
[0043] Preparation Example 3P 1 ,P 2 ,P 3 Both are PNB, P 4 Preparation of compound 2a for H
[0044] Add 2L of anhydrous acetonitrile into the reaction flask, cool to -20°C under the protection of dry nitrogen, add compound 3(P 1 is PNB) 59.5g, compound 4 (P 2 ,P 3 Both are PNB) 69.7g and diisopropylamine 12.1g, react at -15°C for 4h, pour the reaction solution into 2.5L ice water, extract with dichloromethane (1L×3), combine the organic phases, and use Saturated brine (1L×3), dried over anhydrous sodium sulfate, filtered, and concentrated to give 64.5g of compound 2a (P 1 ,P 2 ,P 3 Both are PNB) solids.
[0045] P 1 ,P 2 ,P 3 The preparation of compound 2a, which are all other carboxyl protecting groups, can refer to the method described in Preparation Example 3.
[0046] The present invention will be further described below in conjunction with examples, but these examples do not constitute any limitation to the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com