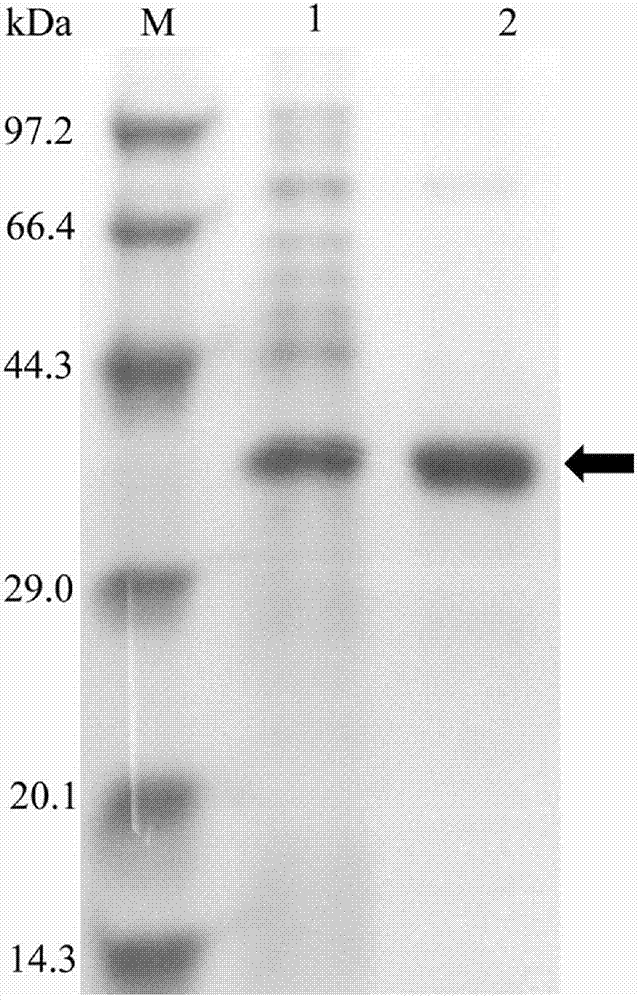
Novel esterase and its application
A new type, esterase technology, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Establishment of metagenomic library and acquisition of positive clones, gene cloning and expression
[0049] 1. Extraction of total DNA:
[0050] Weigh 10g of Turpan soil sample, add 13.5mL DNA extraction buffer (0.1M Tris, 0.1M EDTA-Na, 0.1M Na 3 PO 4 , 1.5M NaCl, 1%CTAB, pH 8.0), shake vigorously for 5min, add 1.5ml 20%SDS, bathe in 65℃ water for 2h, centrifuge at 5000rpm for 5min, take the supernatant, extract twice with equal volume of chloroform, and centrifuge at 16000rpm 10min, take the supernatant, add 0.6 times the volume of isopropanol, place at room temperature for 2h, centrifuge at 16000rpm for 20min, discard the supernatant, add 10mL of pre-cooled 70% ethanol to the precipitate, centrifuge at 16000rpm for 10min, collect the DNA precipitate, air-dry, and use an appropriate amount of supernatant Dissolved in pure water.
[0051] 2. DNA purification by kit method: follow the instructions of the gel recovery kit.
[0052] 3. Metagenomic electroph...
Embodiment 2
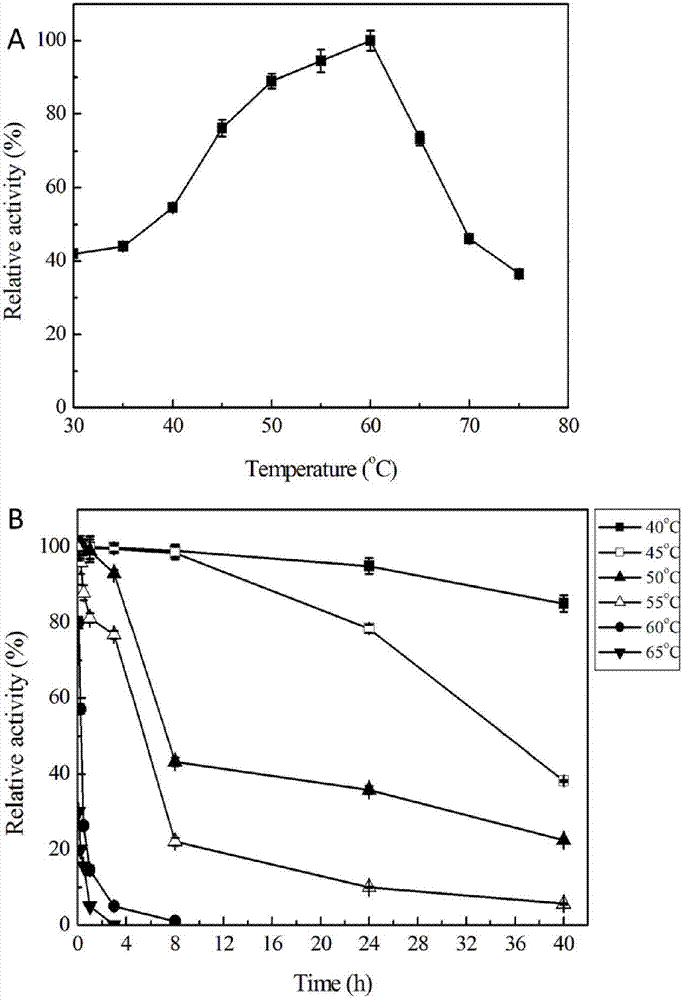
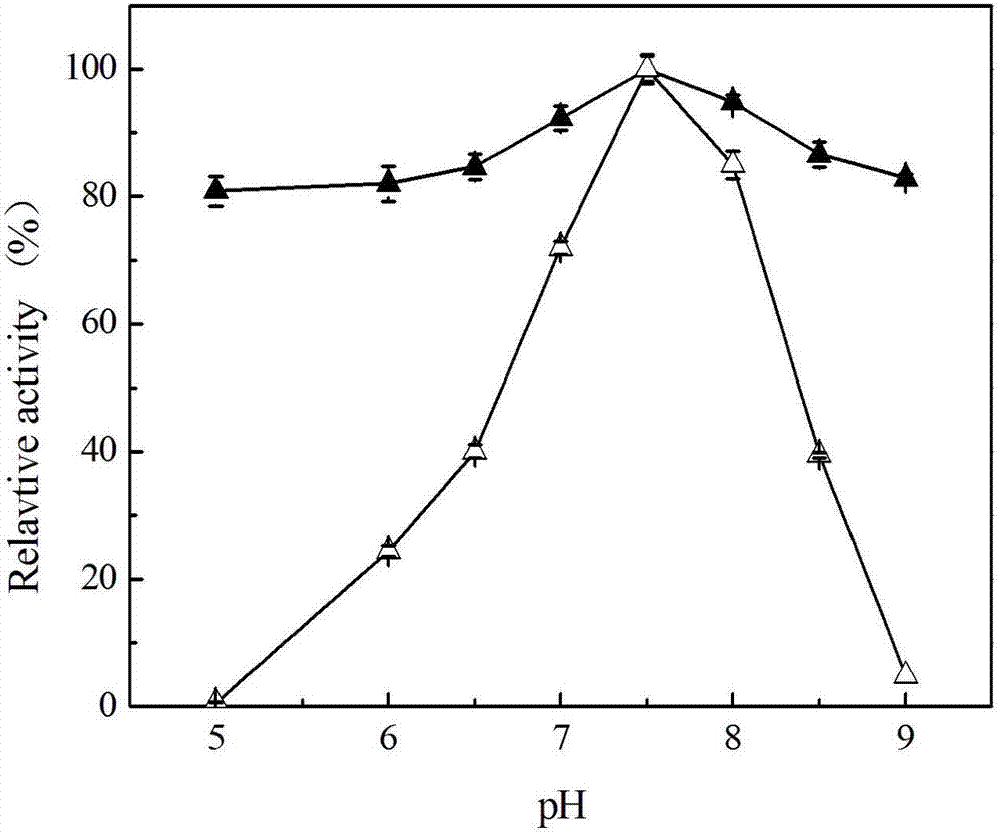
[0072] The enzymatic property research of embodiment 2 recombinant esterase Est112
[0073] 1. Optimum reaction temperature and thermal stability of recombinant esterase
[0074] The crude enzyme solution of recombinant esterase is subjected to enzymatic reaction at 30-75°C, the reaction system is 410 μL, and its composition is 400 μL potassium phosphate solution containing 40 μM p-nitrophenyl acetate (pH7.5) and 10 μL enzyme solution. The reaction system was reacted at 60° C. for 4 minutes, and then the absorbance of p-nitrophenol released during the process was measured at a wavelength of 405 nm, and a blank control without adding enzyme solution was made at the same time. The enzyme activity was measured to obtain the optimum reaction temperature (the highest enzyme activity was recorded as 100%). In 50mM potassium phosphate buffer (pH 7.5), the recombinant esterase enzyme solution was placed at different 40°C, 45°С, 50°C, 55°С, 60°С and 65°C for 40 hours, and the residua...
Embodiment 3
[0077] Example 3 Degradability determination of recombinant esterase Est112 to AHLs
[0078] 1. Reaction system
[0079] Add 1 μmol of C4 -HSL,C 6 -HSL,C 8 -HSL,C 10 -HSL,C 12 -HSL and 3OC 8 - Dissolve HSL in 50 μL of methanol, and add 950 μL of 50 mM potassium phosphate buffer to prepare 1 mM AHLs substrate solution, mix 31 μg of recombinant enzyme powder with 1 ml of 1 mM substrate, react at 30°С for 30 minutes, and perform HPLC Detection, while fire extinguishing enzyme powder as a blank control.
[0080] 2. HPLC analysis
[0081] The chromatographic conditions are as follows:
[0082]
[0083] Through HPLC detection analysis, recombinant esterase to C 4 -HSL,C 6 -HSL,C 8 -HSL,C 10 -HSL,C 12 -HSL and 3OC 8 The degradation rates of -HSL reached 64.7%, 96.2%, 100%, 100%, 100%, and 94.3%, respectively, indicating that it has broad substrate specificity and high ability to degrade AHLs (the degradation profile is shown in 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com