Application of collagen matrix as tissue engineering scaffold
A tissue engineering scaffold and collagen matrix technology, applied in medical science, prosthesis, etc., can solve the problems of poor mechanical properties and fast degradation, and achieve the effects of enhancing performance, improving flexibility, and increasing mechanical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, preparation collagen matrix
[0046] (1) Producing collagen with the original tissue of animals, the specific steps are as follows:
[0047] 1. Remove collagen-rich isolated tissue (pig skin, cowhide, Achilles tendon, etc.) mechanically to remove impurities such as epidermis, muscle, and fat, and then wash it with tap water and pure water after crushing to obtain collagen tissue;
[0048] 2, the dilute alkali solution (mass and number ratio of preparation pH9.5, sodium hydroxide: sodium bicarbonate: water=0.5: 7.5: 92) and alcoholic substance aqueous solution (mass and number ratio, ethanol: Virahol: water =7:4:89);
[0049] 3. Wash the animal tissue obtained in the first step with the above prepared dilute alkali solution and aqueous alcohol solution in sequence to degrease it to obtain crude collagen;
[0050] 4. Dissolving the crude collagen product in a dilute formic acid solution with a pH of 6.0;
[0051]5. Add complex protease to the above soluti...
Embodiment 2
[0062] Embodiment 2, preparation collagen matrix
[0063] (1) Refining commercially available collagen, the specific steps are as follows:
[0064] 1. Dissolve commercially available collagen in dilute hydrochloric acid solution with pH 5.8;
[0065] 2, add composite protease in above-mentioned solution, the weight ratio of collagen and composite protease is 500: 1, and composite protease is made up of three kinds of proteases of activity ratio: pepsin: trypsin: papain=1: 1: 0.5, 34 Cultivate with stirring at ℃ for 11 hours;
[0066] 3. Centrifuge and collect the supernatant, add NaCl to the supernatant to make the concentration reach 2mol / L, stir at 8°C for 12 hours, and collect the obtained precipitate;
[0067] 4. Dissolve the precipitate in an aqueous solution of 0.3% acetic acid, and collect the supernatant by centrifugation;
[0068] 5. Add NaCl to the supernatant to make the concentration reach 1mol / L, and stir to obtain a precipitate; repeat the operation 4 times, a...
Embodiment 3
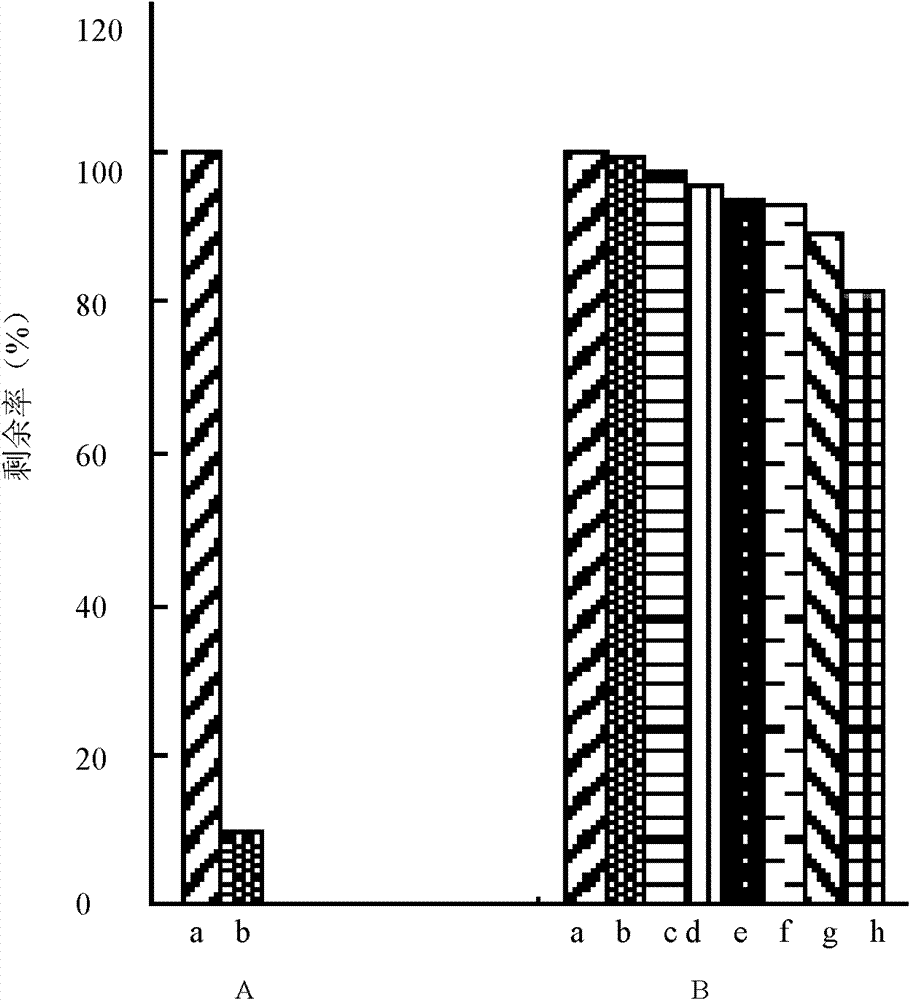
[0077] Embodiment 3, the enzymatic stability of collagen matrix
[0078] The rate of enzymatic degradation was determined by enzymatic degradation of collagen matrix.
[0079] The specific method is as follows: take fresh DMEM culture solution to prepare a collagenase solution with a concentration of 5 CDU / ml, and store it in cold storage; take a 5 mg collagen matrix sample, and package it with a plastic film 60 C 0 Sterilization; put the sample into 1ml type I collagenase solution, incubate at 37°C, replace the collagenase solution every day; take samples at 1h, 1.5h, 4h, 1d, 2d, 3d, 6d, 9d time points, each group 3 samples; add 200μl 0.25mol / L EDTA solution to the regularly sampled solution to terminate the digestion process; take out the sample with a small corner of the sample with tweezers, wash it with phosphate buffer (pH7.2) first, and then wash it with deionized water , placed in a clean small tube, and stored at -20°C for more than 24 hours; the sample was freeze-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com