Spiro-fluorene-9,9-xanthene bipolar luminescent material, its preparation method and its application method
A luminescent material, xanthene fluorene technology, applied in the manufacture of semiconductor/solid-state devices, electrical components, circuits, etc., to achieve good photoelectric performance, good thermal stability, and the effect of making stable electroluminescent devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
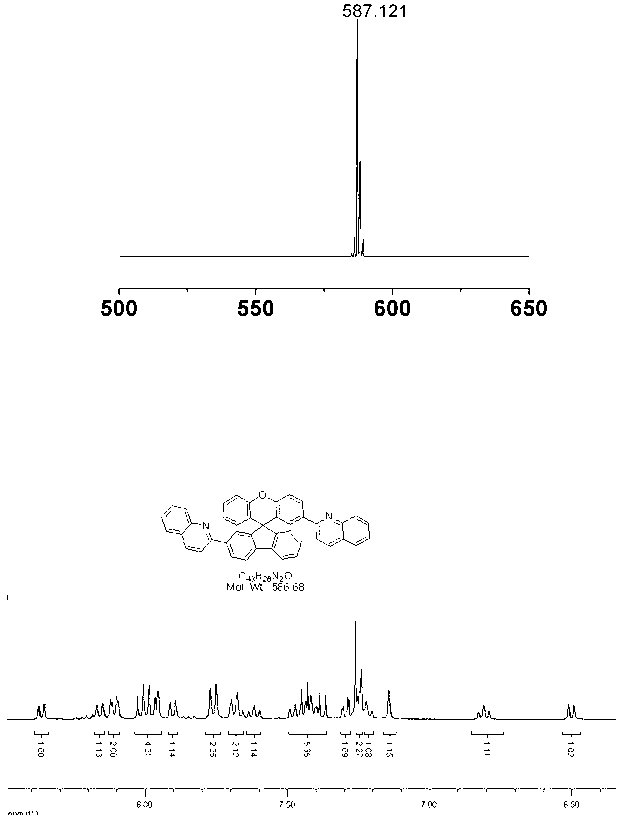
[0040] Example 1: spiro-9,9-xanthene fluorene
[0041] 9-Fluorenone (3.474 g, 19.3 mmol), phenol (18 g, 191.4 mmol) and methanesulfonic acid (5.0 mL, 77.2 mmol) were heated to reflux at 150°C for 24 hours under the protection of light and nitrogen. It was then cooled to room temperature, neutralized to alkaline with aqueous sodium hydroxide solution, and filtered with suction to obtain a crude product of spiro-9,9-oxanthene fluorene with high purity (yield, 85%).
Embodiment 2
[0042] Example 2: 2'-Acetyl-spiro-9,9-xanthene fluorene
[0043] Spiro-9,9-xanthene fluorene (3.3200 g, 10 mmol) and 50 mL of dichloromethane were stirred and dissolved in an ice-water bath under nitrogen protection for 20 minutes, and then acetic anhydride (0.94 mL, 10 mmol) was The constant pressure dropping funnel was slowly added dropwise to the reaction bottle, and reacted for 24 hours. The reaction mixture was slowly added to an ice-water mixture to quench, extracted with dichloromethane, the combined organic phases were dried over anhydrous magnesium sulfate, concentrated by suction filtration under pressure. Using a mixture of ethyl acetate and petroleum ether as the eluent, a white product was obtained by column chromatography (yield, 47%).
Embodiment 3
[0044] Example 3: Synthesis of 2'-isoquinolinyl-spiro-9, 9'-oxanthrene:
[0045] Under the protection of nitrogen, 2-acetyl-9,9'spiroxanthene fluorene (1.1120 g, 3 mmol) and anthranilaldehyde (1.8200 g, 15.0 mmol) were successively added to the reaction flask, and saturated potassium hydroxide was injected Solution 5mL and ethanol 25mL were refluxed overnight at 90°C, washed with water, extracted with dichloromethane, combined organic phases, dried over anhydrous magnesium sulfate, concentrated by rotary evaporator, using petroleum ether and dichloromethane as eluents, column The product was obtained by chromatography (yield, 44%). GC-MS (EI) m / z 459 [M + ];1H NMR (400 MHz, CDCl 3 ): δ (ppm) 8.13 (dd, J = 8.6, 1H), 8.0 (m, 2H), 7.84 (d, J = 7.63, 2H), 7.71 (d, J = 8.04, 1H), 7.64 (t, J = 6.98, 1H), 7.41 (m, 5H), 7.28 (s, 1H), 7.22 (m,5H), 7.08 (s, J = 2.16, 1H) , 6.80 (s, J = 8.12, 1H) , 6.44 (s, J = 7.81, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com