Extraction method and application of falcate dolichos root or leaf glucoside A and total saponins of falcate dolichos root or leaf
A technology of cannabis medicine and glycoside, which is applied in the field of preparation of cannabinoid glycoside A and total saponins of cannabis medicine, can solve problems such as adverse reactions, failure to prevent tissue damage, joint destruction and deformity, and achieve low price and significant anti-immunity Inflammatory action, definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of cannabinoid total saponins and cannabinoid glycoside A
[0050] The preparation of the total saponins of the cannabis medicine: get 250g of the cannabis medicine medicinal material granules, place in a 5L round-bottomed flask, add 10 times the weight of the cannabis medicine medicinal material granules with a volume concentration of 60% ethanol, soak for 2 hours, and extract by reflux in a water bath. Extract three times, and the extraction time is 2, 1.5, and 1 hour respectively, and the filtrates are combined. The filtrate recovered ethanol under reduced pressure until it had no alcohol smell, and then dried it under reduced pressure at 60°C to dryness to obtain about 55 grams of total cannabinoid saponins (as determined by HPLC, the content of cannabinoid glycoside A was ≥52%).
[0051] The preparation method of cannabinoid glycoside A: take 250g of cannabis medicinal material granules, place them in a 5L round-bottomed flask, add 10 times the weight o...
Embodiment 2
[0053] The structure identification of cannabinoid glycoside A extracted in Example 1 was carried out.
[0054] Cannabinoid A, the compound has the formula C 37 h 58 o 11 , white powder, easily soluble in methanol, ethanol and water; melting point: m.p.295-297℃;
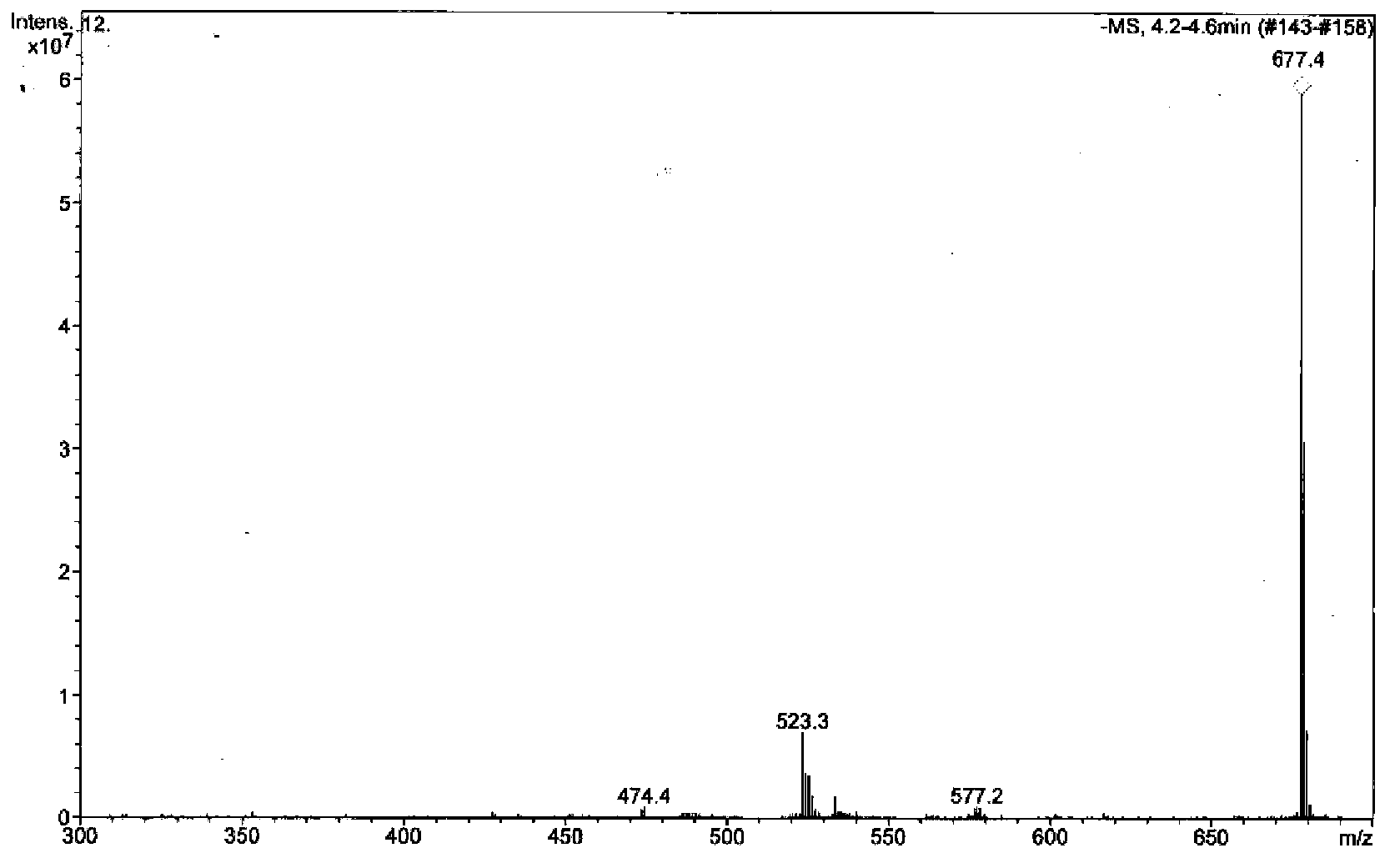
[0055] MS data (combined with figure 1 ): EI-MS (70eV) m / z, [M] - 677.4 (100); IR: 3400, 2930, 1695, 1445, 1260, 1165 cm -1 , indicating that there are phenolic hydroxyl groups, carbonyl groups and double bonds;
[0056] UV: Carry out full-band scanning under ultraviolet 190-400nm, and find that there is maximum absorption at 195nm;
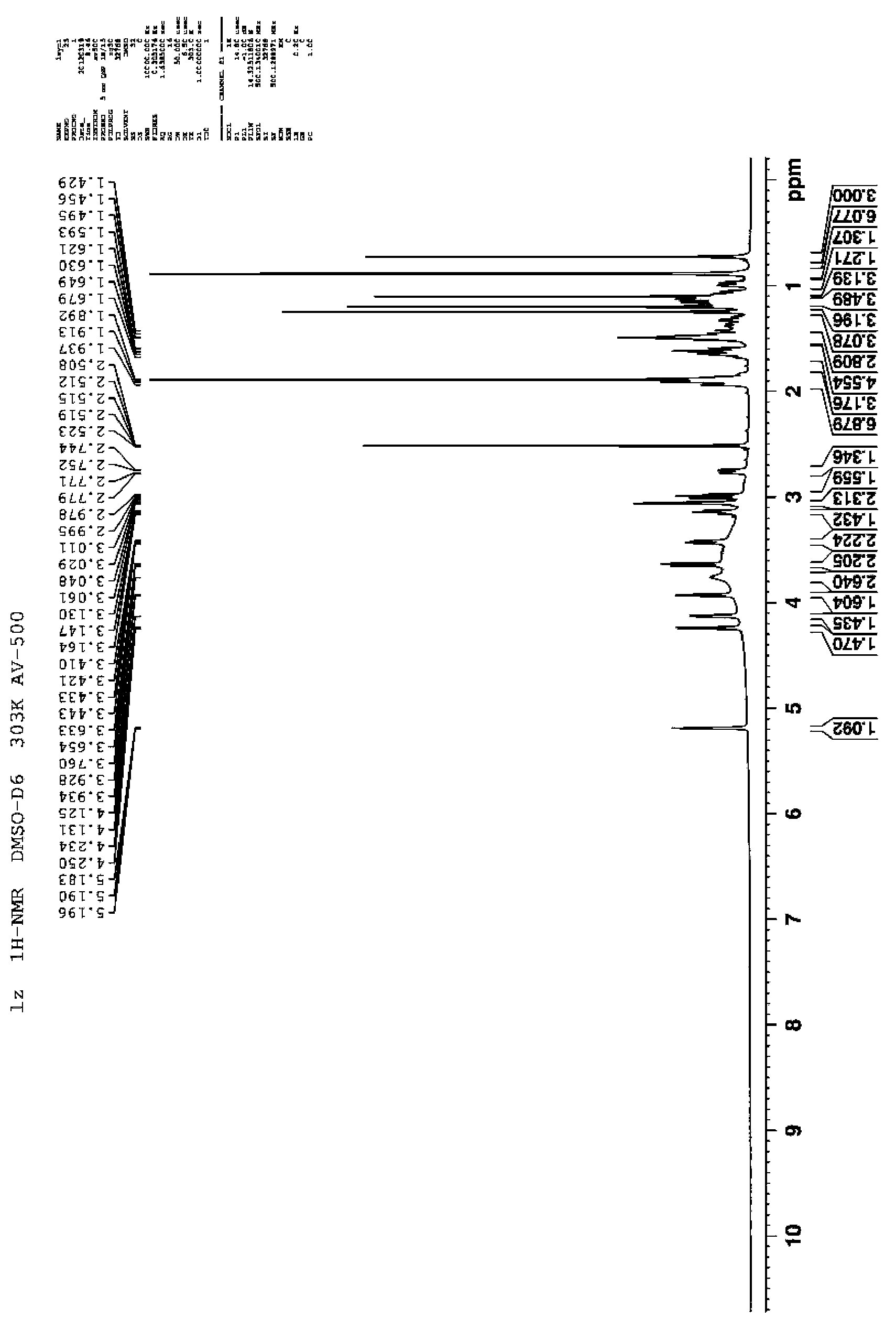
[0057] H NMR spectroscopy (combined with figure 2 ): 1 H-NMR (DMSO-d6, 500MHz) δ: 5.5654 (1H, m, J=10.08Hz, H-12), 4.7265(1H, d, J=4.12, H-3), 4.5022(1H, m, J =9.95Hz, H-2), 2.0242(s, 2H, H-2), 1.6229 (1H, s, H-6), 1.0279, 0.8144, 0.9455;
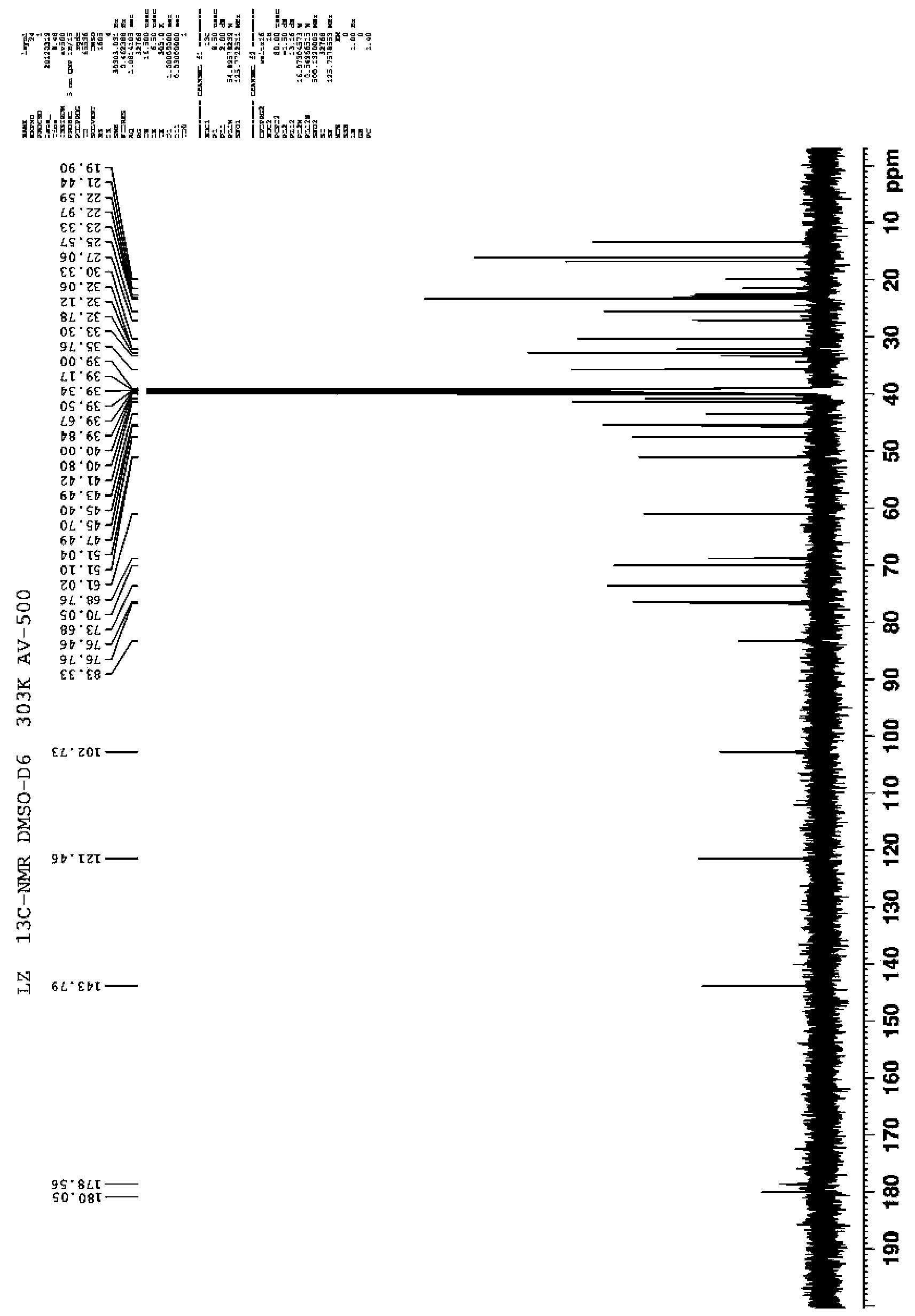
[0058] NMR carbon spectrum: 13 C-NMR (DMSO-d6, 500MHz) data are shown in Table 1;
[0059] According to the above physical and chemic...
Embodiment 3
[0065] Content determination of cannabinoid glycoside A and total saponins of cannabinoids
[0066] 1. Chromatographic conditions
[0067] Agilent 1100 high performance liquid chromatography, the chromatographic column is Agilent TC-C18 (4.6×250 nm, 5 μm); detection wavelength: 203 nm; mobile phase: A is CH 3 CN, B is 0.1% phosphoric acid aqueous solution, gradient elution [0~8 min, 30%~40% A, 8~15 min, 40%~65% A, 15~20 min, 65%~75% A], Flow rate: 1.0 mL·min -1 , column temperature: 30°C, injection volume: 20 μL.
[0068] 2. Preparation of test solution
[0069] 2.1 Configuration of standard solution: Accurately weigh 2.4 mg of cannabinoid glycoside A standard dried to constant weight, add methanol to a 10 mL volumetric flask, and shake well to obtain 0.24 mg·mL -1 cannabinoid glycoside A standard.
[0070] 2.2 Preparation of cannabinoid glycoside A and cannabinoid total saponins sample solution:
[0071] Precisely weigh 12.8 mg of the total saponin sample of the cannabi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com