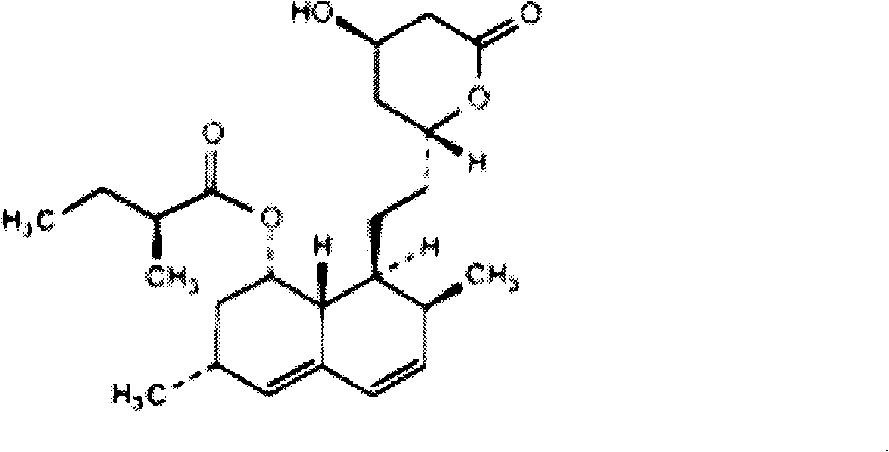
Bionic lovastatin nano-structured lipid carrier and preparation method thereof
A nano-lipid carrier, lovastatin technology, used in microcapsules, emulsion delivery, nanocapsules, etc., can solve the problems of low drug loading, hemolysis, poor water solubility, etc., to improve poor oral absorption and reduce toxicity. The effect of reducing side effects and toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of biomimetic lovastatin nano-lipid carrier
[0023] prescription:
[0024]
[0025] Preparation process: Weigh the prescribed amount of lovastatin, soybean lecithin, arachidonic acid, cholesterol oleate, triolein, and cholesterol, dissolve in ethanol, and evaporate under reduced pressure to obtain a dry lipid film. Add hydration medium and hydrate in a constant temperature water bath at 30°C for one hour, ultrasonically disperse the hot colostrum, then perform probe ultrasonication (300W, 200s), then filter and sterilize with a 0.22μm microporous membrane, put it in a sterile bottle and seal it for storage.
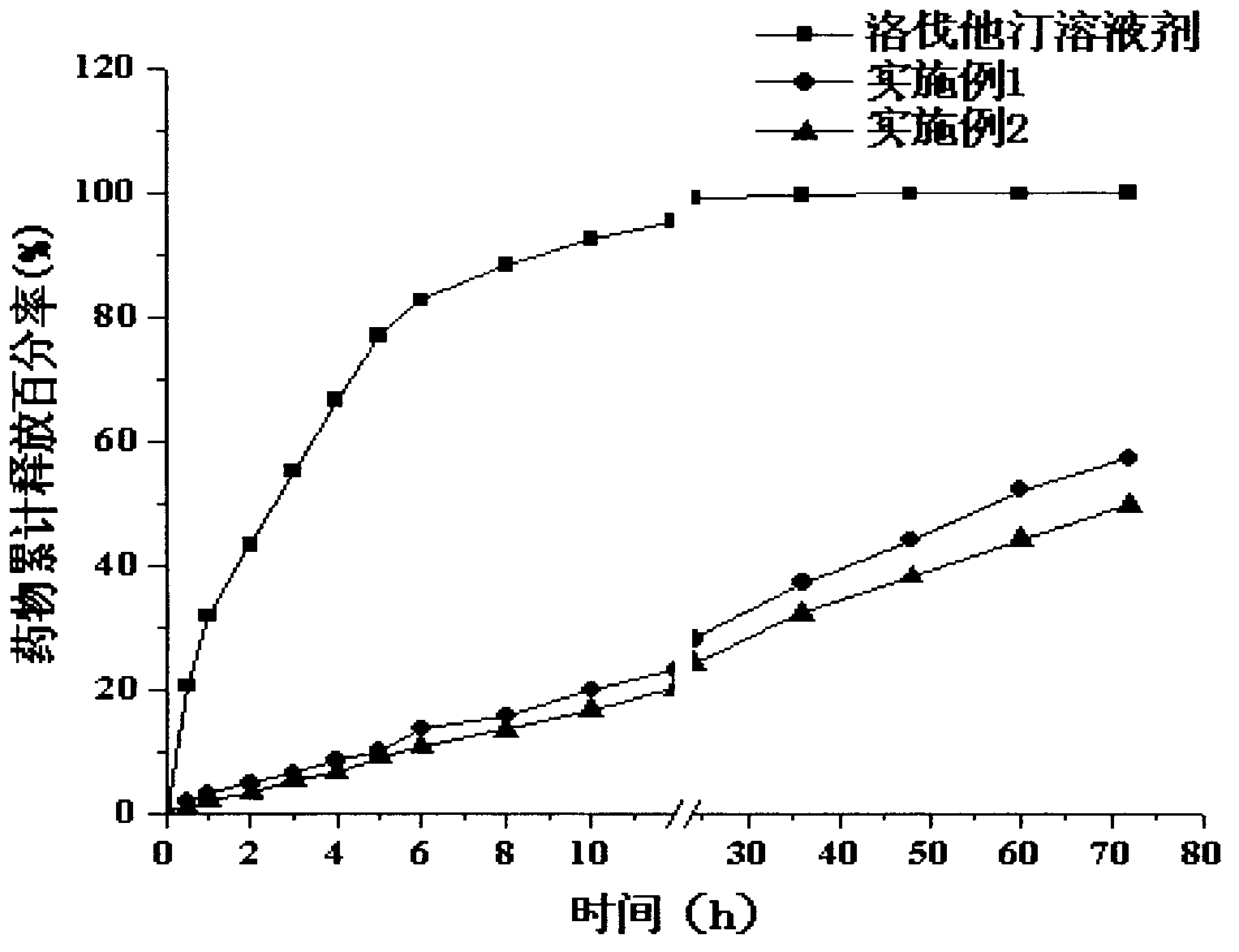
[0026] The prepared lovastatin nano-lipid carrier has a particle size of 128.3nm, a polydispersity index (PI) of 0.246, an encapsulation efficiency of 91.62%, a drug loading capacity of 3.4%, and a Zeta potential of -34.2mV. release curve as figure 1 shown.
Embodiment 2
[0027] Embodiment 2: Preparation of biomimetic lovastatin nano-lipid carrier
[0028] prescription:
[0029]
[0030] Preparation process: Weigh the prescribed amount of lovastatin, egg yolk lecithin, arachidonic acid, cholesterol oleate, triolein, and cholesterol, dissolve in chloroform, and dry lipid film by rotary evaporation under reduced pressure. Add hydration medium and hydrate in a constant temperature water bath at 25°C for one hour, ultrasonically disperse the hot colostrum, then perform probe ultrasonication (300W, 200s), then filter and sterilize with a 0.22μm microporous membrane, put it in a sterile bottle and seal it for storage.
[0031] The prepared lovastatin nano-lipid carrier has a particle size of 135.5nm, a polydispersity index (PI) of 0.253, an encapsulation efficiency of 91.34%, a drug loading capacity of 3.1%, and a Zeta potential of -32.6mV. release curve as figure 1 shown.
Embodiment 3
[0032] Embodiment 3: Preparation of biomimetic lovastatin nano-lipid carrier
[0033] prescription:
[0034]
[0035]
[0036] Preparation process: Weigh the prescribed amount of lovastatin, dioleoyl lecithin, arachidonic acid, cholesterol oleate, triolein, and cholesterol, dissolve in dichloromethane, and dry lipid by rotary evaporation under reduced pressure film. Add hydration medium and hydrate in a constant temperature water bath at 40°C for one hour, ultrasonically disperse the hot colostrum, then perform probe ultrasonication (300W, 200s), then filter and sterilize with a 0.22μm microporous membrane, put it in a sterile bottle and seal it for storage.
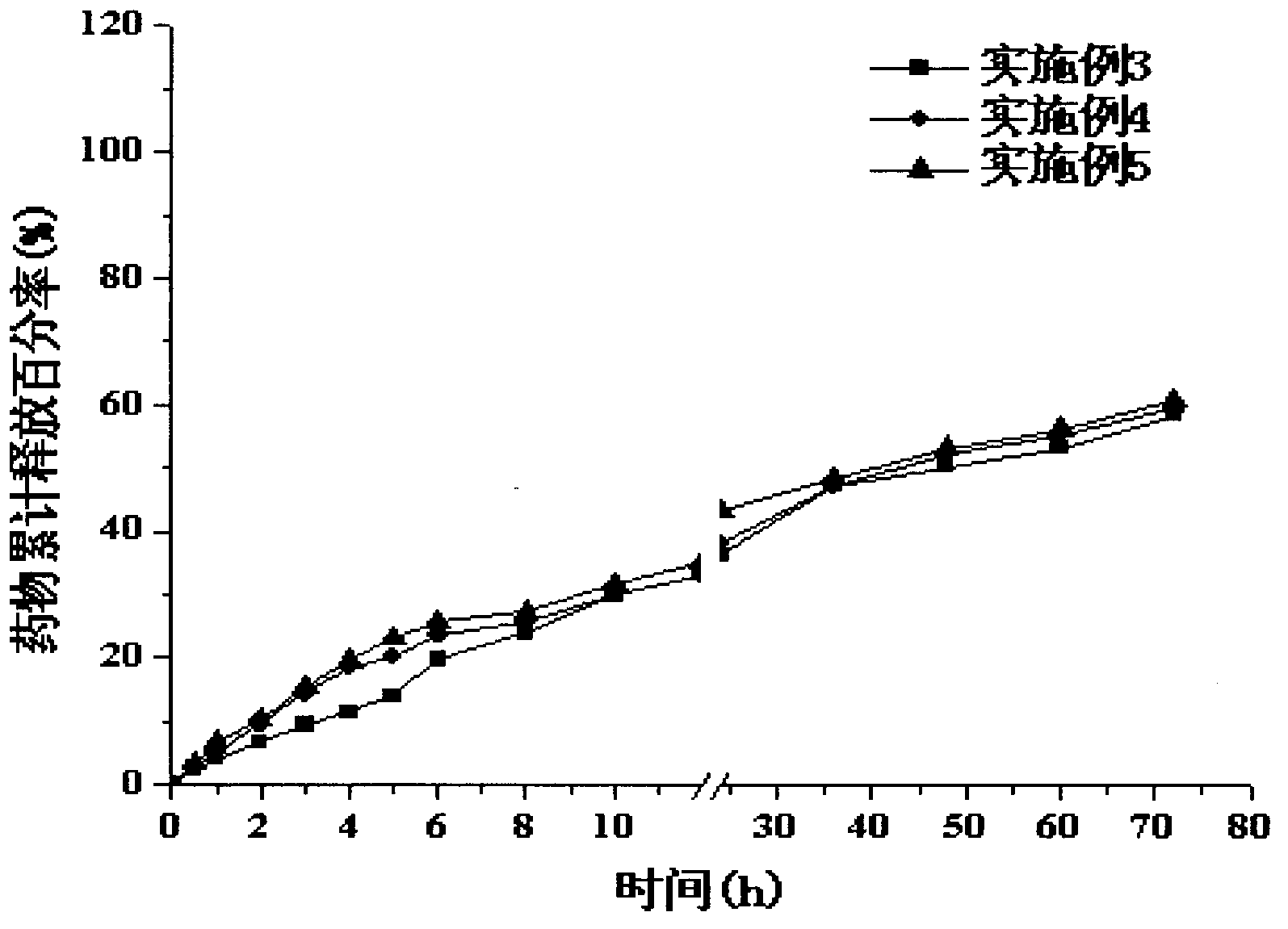
[0037] The prepared lovastatin nano-lipid carrier has a particle size of 123.2nm, a polydispersity index (PI) of 0.227, an encapsulation efficiency of 92.51%, a drug loading capacity of 2.8%, and a Zeta potential of -37.4mV. release curve as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com