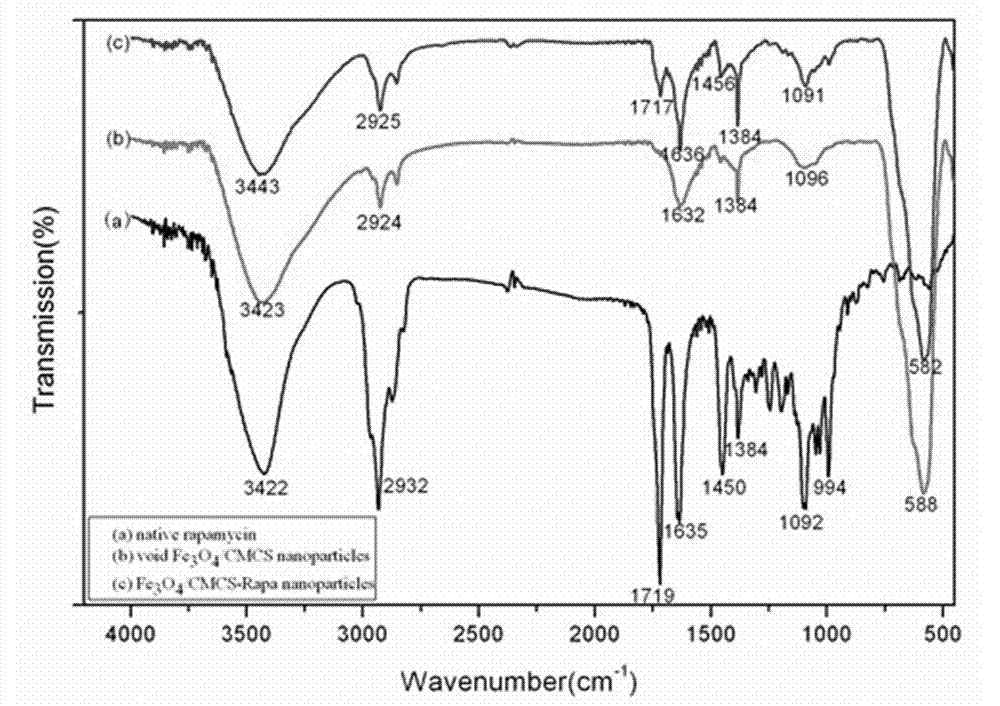
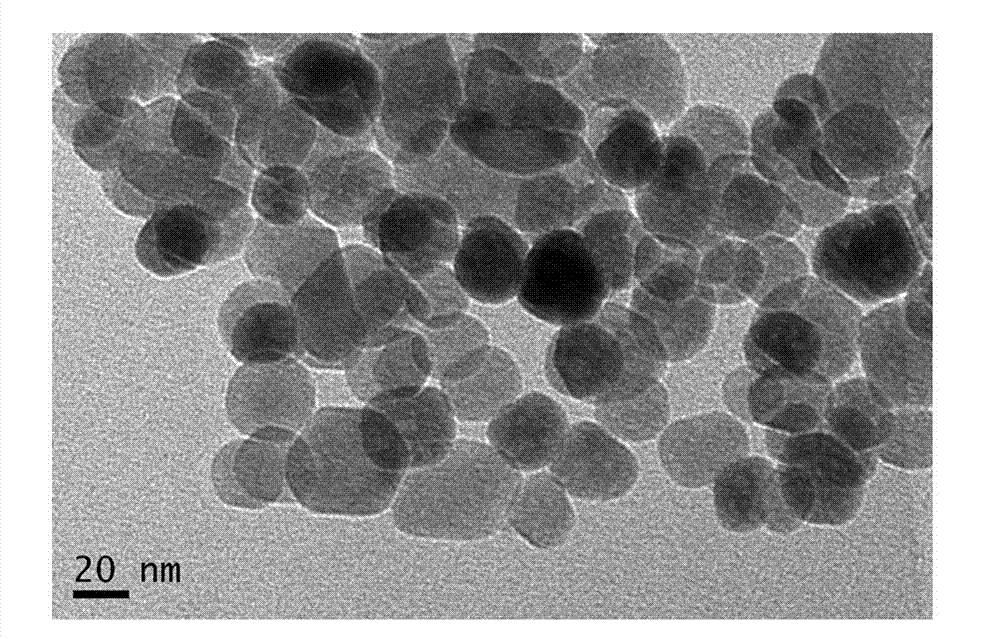
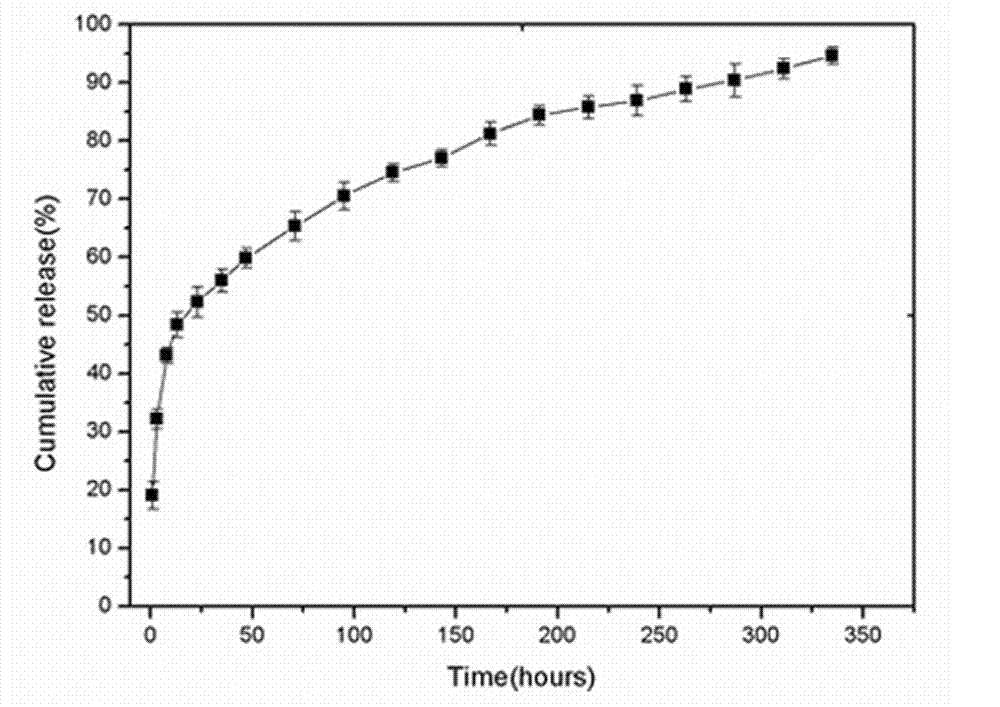
Method for preparing rapamycin/magnetic carboxymethyl chitosan nano drug-loaded microspheres
A technology of carboxymethyl chitosan and rapamycin is applied in the application field of biofunctional materials in the field of biomedicine, which can solve the problem of inability to solve normal tissue damage, failure to achieve optimal therapeutic effects, and drug-loaded microspheres. problems such as poor targeting, to achieve the effect of strengthening the effect of targeted therapy, low toxicity administration, and high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Accurately weigh 0.4g of carboxymethyl chitosan (CMCS) and dissolve in 20ml of deionized water, stir to dissolve and filter, then add 0.4g of Fe 3 o 4 Nanoparticles, ultrasonic 5min to make them evenly dispersed; add 60ml of liquid paraffin oil (containing 1.8ml span-80) into a 250ml three-neck bottle, slowly add carboxymethyl chitosan solution drop by drop into the liquid paraffin oil while stirring , the mixture was sonicated for 30 minutes, fully stirred at 500 rpm for two hours, slowly added dropwise with 2ml of 25% glutaraldehyde solution, and stirred at 500 rpm for 2 hours. For magnetic separation, first wash thoroughly with petroleum ether for 2-3 times, then continue to wash with acetone for 3 times, so as to obtain clean nanoparticles, and dry them in a vacuum oven at 60°C for 12 hours to obtain Fe 3 o 4 / CMCS nanoparticles, spare.
[0045] (2) Accurately weigh 100mg of Fe 3 o 4 / CMCS nanoparticles, dispersed in 14ml of ultrapure water; weigh 11.9mg of r...
Embodiment 2
[0047] Accurately weigh the Fe prepared by 100mg embodiment 1 3 o 4 / CMCS nanoparticles were dispersed in 15ml of ultrapure water; 10mg of rapamycin (Rapa) was weighed and dissolved in 1ml of acetonitrile. Then the Rapa solution was slowly added dropwise to the Fe 3 o 4 / CMCS nanoparticle dispersion, stirred overnight with a magnetic stir bar at 600 rpm. First wash with ultrapure water, then magnetically separate; repeat the steps of ultrapure water washing and magnetic separation for 3 times, dry in vacuum freeze-drying equipment for 15 hours, and pulverize to obtain Fe 3 o 4 / CMCS-Rapa nanoparticles. Produced Fe 3 o 4 / CMCS-Rapa nanoparticles have a uniform particle size distribution, and the drug-loaded nanoparticles are regular spherical. The average encapsulation efficiency is 52.53%, and the drug-loading capacity is 5.2%.
Embodiment 3
[0049] Accurately weigh the Fe prepared by 100mg embodiment 1 3 o 4 / CMCS nanoparticles were dispersed in 15ml of ultrapure water; 12.5mg of rapamycin (Rapa) was weighed and dissolved in 1ml of acetonitrile. Then the Rapa solution was slowly added dropwise to the Fe 3 o 4 / CMCS nanoparticle dispersion, stirred overnight with a magnetic stirrer at 1500 rpm. Wash with ultrapure water first, and then magnetically separate; repeat the steps of ultrapure water washing and magnetic separation 5 times, freeze-dry for 12 hours, and grind to obtain Fe 3 o 4 / CMCS-Rapa nanoparticles. Produced Fe 3 o 4 / CMCS-Rapa nanoparticles have a uniform size distribution, and the drug-loaded nanoparticles are regular spherical. The average encapsulation efficiency is 46.36%, and the drug-loading capacity is 6.32%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com