Method for improving thermal stability of GH10 xylanase through N-terminal replacement
A xylanase and heat-resistant technology, applied in the field of bioengineering, can solve the problems of difficult thermal stability, poor thermal stability, restricting the application of xylanase, etc., and achieve the effect of improving the optimum temperature and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Construction of mutant enzyme
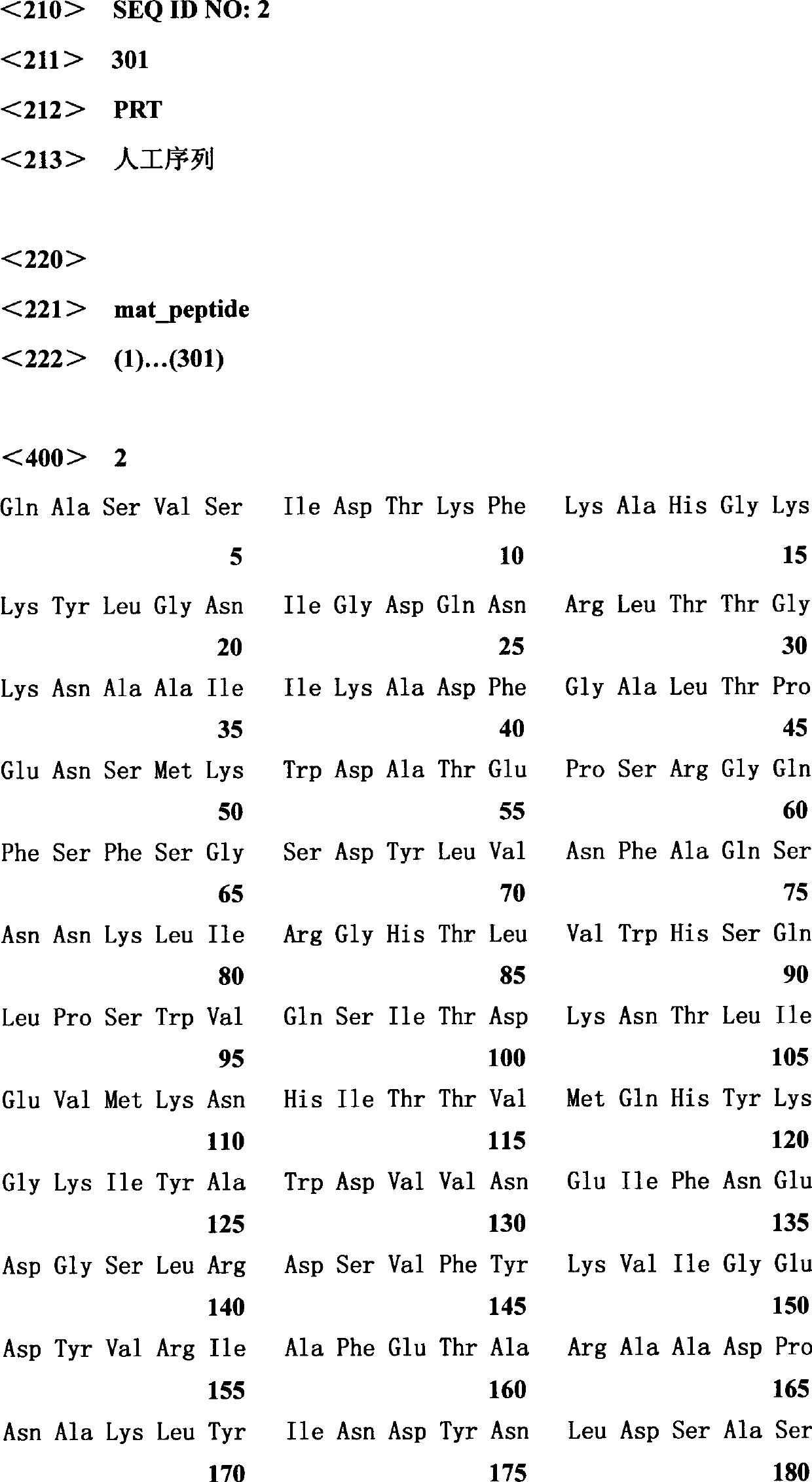
[0017] The T vector (pUCm-T-Aus xyn10A) containing the Aus Xyn10A gene was extracted, and the mature peptide primer Xyn10A-F1 and the primer XynCHNR1 of the Aus Xyn10A gene were used as a template for the first round of PCR. The reaction conditions were 94°C for 5 min; 2 cycles, 94°C 30s, 45°C 30s, 72°C 70s; 28 cycles of 94°C 30s, 55°C 30s, 72°C 30s; 72°C 10min; 10°C storage; then pUCm-T-Ausxyn10A as template, Use large primer PCR to use Xyn10A-R1 (5'-GCGGCCGCCTAGAGAGCATTTGCGATAG-3') and the first round of PCR products as primers for the second round of PCR. The reaction conditions are: 94℃ for 5min; 2 cycles, 94℃ for 30s, 45℃ 30s, 72℃70s; 28 cycles of 94℃30s, 55℃30s, 72℃70s; 72℃10min; 10℃ storage. Two rounds of PCR amplification products were analyzed by 1% agarose gel electrophoresis, the gel was tapped and the target band was recovered and ligated with pUCm-T vector (pUCm-T-ATx10AM), transformed into JM109, and sent to Shanghai...
Embodiment 2
[0018] Example 2 Construction of an expression plasmid containing the gene encoding the mature peptide of ATx10AM
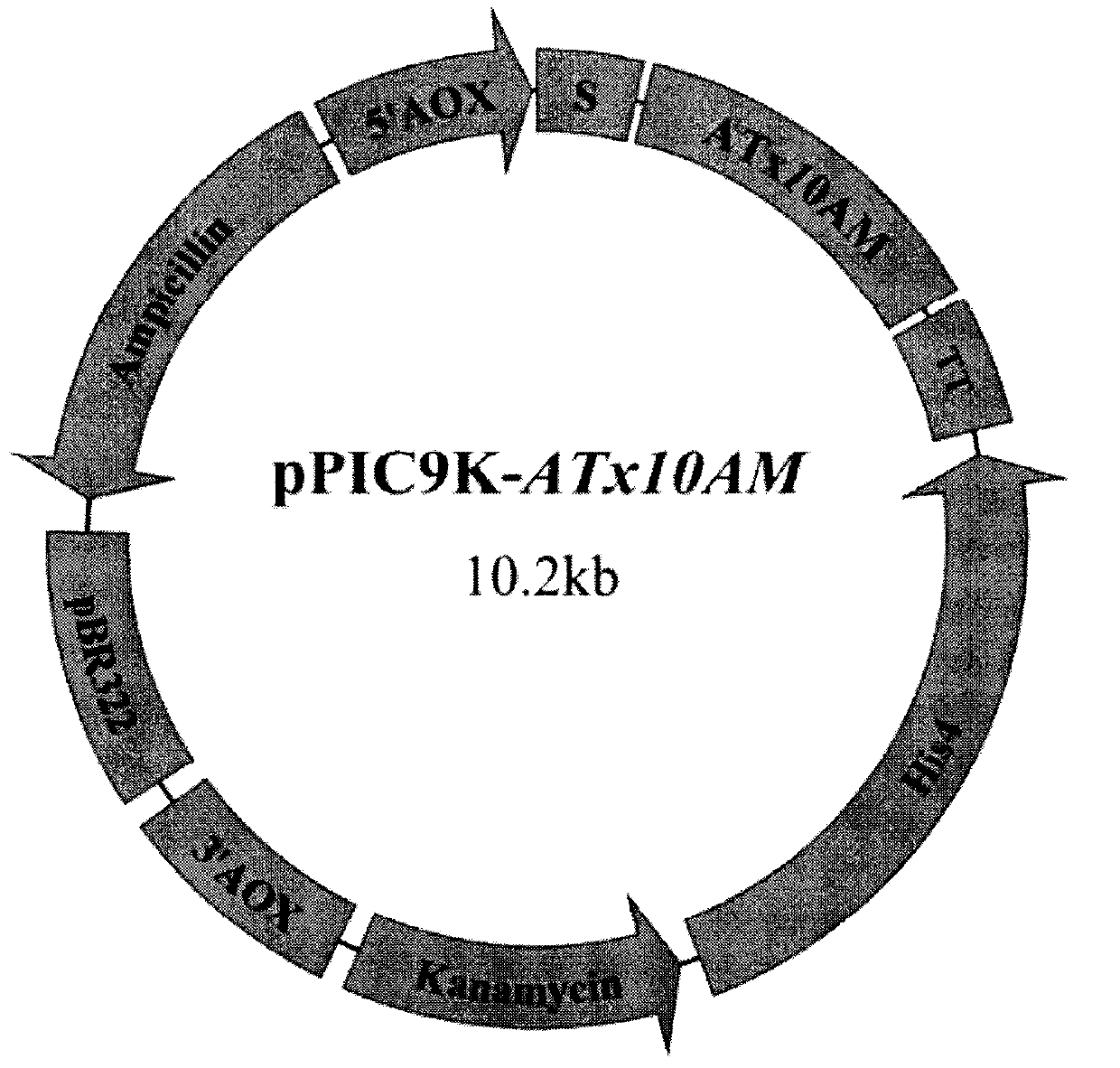
[0019] Use EcoR I and Not I to double-enzyme digestion of the target gene recovered by tapping and pPIC9K. The digestion time is 4 hours. The digested product recovered from tapping is ligated overnight (>12h) under the action of T4DNA ligase to obtain the recombinant plasmid pPIC9K. -ATx10AM( figure 1 ), and sequence the recombinant expression plasmid.
Embodiment 3
[0020] Example 3 Construction, expression, product purification and activity determination of GS115 / ATx10AM
[0021] The pPIC9K-ATx10AM was linearized with Sal I, electrotransformed and screened according to the Pichia expression manual, and the high-copy Pichia recombinant GS115 / ATx10AM was obtained. The engineered bacteria was induced with 0.5% methanol for 72 hours, and the recombinant xylanase activity in the fermentation broth measured by DNS method reached 521 IU / mL. The supernatant of the centrifugation is the crude recombinant xylanase enzyme solution, which is concentrated by an ultrafiltration membrane with a molecular weight cut-off of 10kDa, and then purified by DEAE-Sepharose Fast Flow ion exchange chromatography and Sephadex G-75 gel filtration chromatography. SDS-PAGE detected a single band, and showed that the molecular weight of recombinant xylanase was 42kDa. The optimal temperature of the recombinant xylanase is 55°C, which is 10°C higher than the original enz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com