A kind of method that heat reduction prepares graphene
A graphene and vacuum heating technology, applied in chemical instruments and methods, inorganic chemistry, non-metallic elements, etc., can solve the problems of graphite electrochemical performance limitations, inconvenient industrial production, and many oxygen-containing functional groups, and achieve good flame retardation Features, convenient operation, fast response and efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Graphene oxide was prepared by the classic Hummers method: Weigh 1 g of flake graphite and stir it for 10 min in a three-necked bottle filled with 23 mL of concentrated sulfuric acid, and then move the device into an ice bath environment below 4 °C. Slowly add 0.5g of sodium nitrate, 3g of potassium permanganate, and stir in an ice bath for 2h. After the low-temperature oxidation is completed, remove the ice bath, raise the temperature to 35-40°C for medium-temperature oxidation, and keep it for 30 minutes. Add 46~50mL deionized water after the middle temperature oxidation, and carry out high temperature oxidation at 95~98°C for 30min. Subsequently, 140 mL of deionized water and 5 mL of 30% hydrogen peroxide were added to terminate the reaction, and filtered and washed. The graphene oxide product was obtained by repeated washing with deionized water and ethanol for 1 to 3 times, and vacuum drying at 50°C.
[0030] Weigh 100 mg of graphene oxide and ultrasonically disp...
Embodiment 2
[0034] Graphene oxide was prepared by the classic Hummers method, and the method for the alkali metal content in graphene oxide being 0.4% was the same as that described in Example 1.
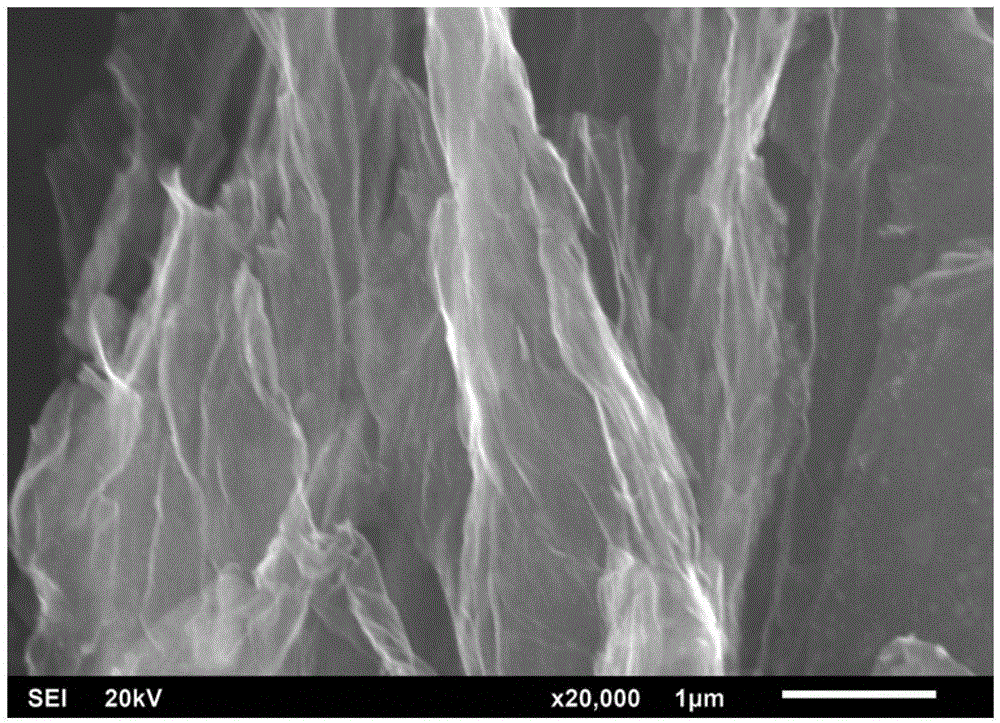
[0035] A bulk graphene oxide sheet with a potassium metal content of 0.4% was ignited by a high-temperature copper rod, and the copper rod was heated to 400 °C and 800 °C by a muffle furnace, respectively. The ignited graphene oxide will undergo a combustion heat reduction process, and finally generate black flake and powder solid graphene. The surface structure of the graphene is loose and porous, and the edge of the graphene in contact with the high-temperature copper rod will burn red, and the inside of the graphene is stable, and no combustion reaction will occur after ignition, which shows that the reduced graphene prepared by the combustion heat reduction method Material has good flame retarding properties. Graphene obtained by igniting copper rods at 800°C has greater loss than graphene...
Embodiment 3
[0038] Graphene oxide was prepared by the classic Hummers method, and the method for the alkali metal content in graphene oxide being 0.4% was the same as that described in Example 1.
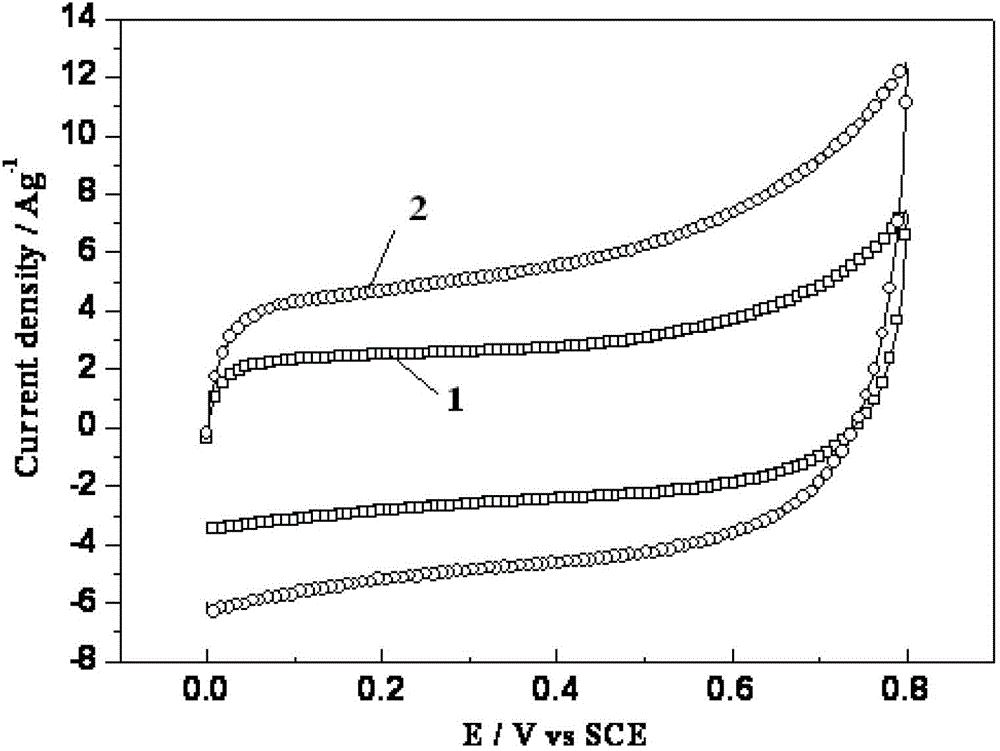
[0039] The bulk graphene oxide sheet with a potassium content of 0.4% was ignited on the outer flame of an alcohol lamp, and a spontaneous combustion reaction occurred. After the reaction is completed, the brown graphene oxide sheet will turn into black powdered graphene, which can still maintain a sheet-like structure, which is soft, fluffy and porous, and the surface structure expands due to the reduction process of combustion heat, reducing the The degree of agglomeration of graphene. At the same time, the combustion heat reduction reaction can greatly reduce the oxygen-containing functional groups on the surface of graphene oxide, which improves the electric double layer capacitance characteristics of graphene.
[0040] The steps and test methods for making electrodes from the graphene mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com