Manufacturing method of high-open-circuit-voltage photovoltaic cell
A manufacturing method, open circuit voltage technology, applied in photovoltaic power generation, final product manufacturing, sustainable manufacturing/processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
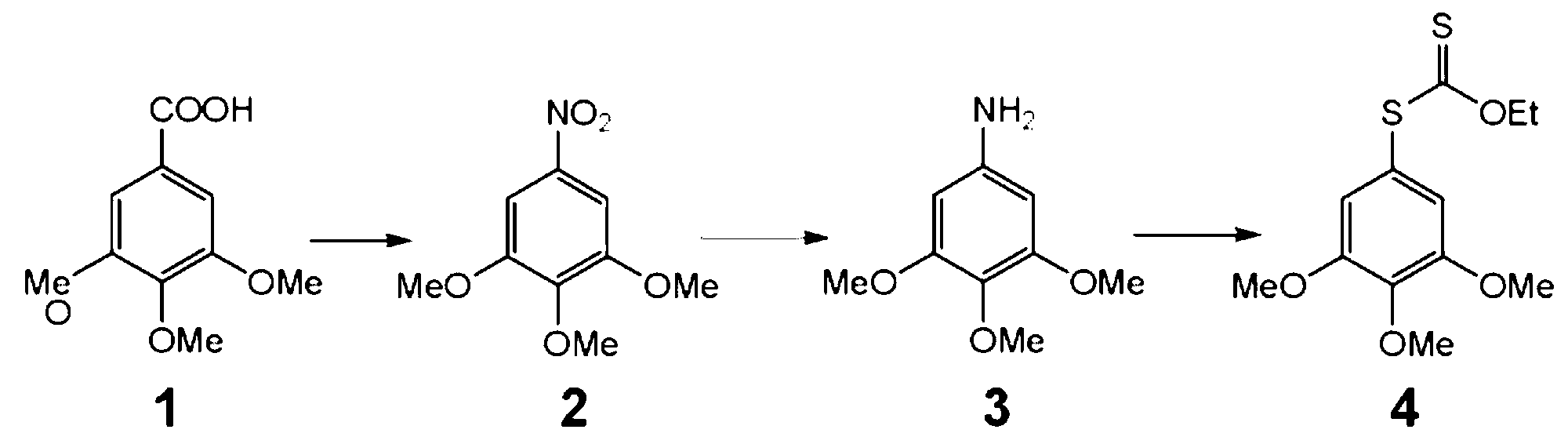
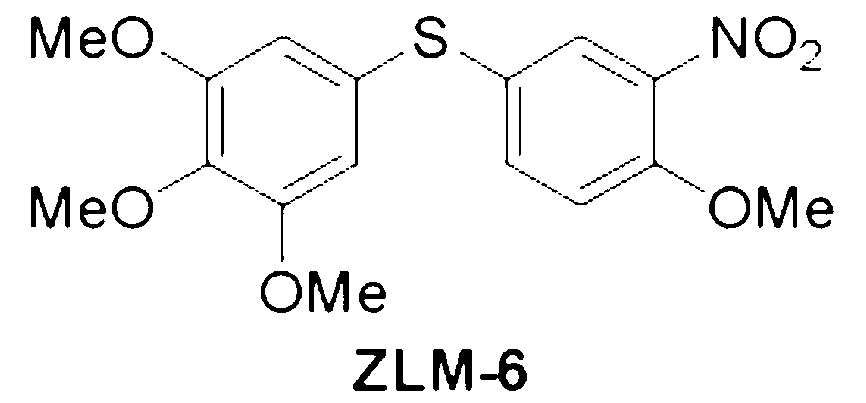
[0073] Preparation of 3,4,5-trimethoxy-1-[(3-nitro-4-methoxyphenyl)thio]benzene (ZLM-6)
[0074]
[0075] Dissolve O-ethyl-S-(3,4,5-trimethoxyphenyl)dithiocarbonate ZLM-1 (2.42g) in THF (32mL), slowly add LiAlH 4 (1.3g), refluxed for 1h, cooled to room temperature, adjusted to pH=5 with 10% HCl, then extracted 20mL×4 with EA, combined the organic phases, dried with anhydrous sodium sulfate for 1h, and then filtered with suction to obtain the filtrate, which was spun After removing the solvent, a yellow oil was obtained. Under nitrogen protection, first add potassium carbonate (1.16g, 8.40mmol, 2.0eq), CuI (400mg, 2.10mmol, 0.5eq) and ethylene glycol (0.49mL, 8.40mmol, 2.0eq) into a 25mL two-necked flask , and then dissolve the yellow oil obtained in the previous step in isopropanol (4.0 mL), then add it to the reaction flask, reflux at 80 ° C for 20 h, cool to room temperature, filter with suction, and wash the filter residue with EA several times to obtain the filtrate. ...
Embodiment 2
[0077] Preparation of 3,4,5-trimethoxy-1-[(3-amino-4-methoxyphenyl)thio]benzene (ZLM-7)
[0078]
[0079] According to Example 1, 1-(3'- Nitro-4'-methoxyphenyl)-2-methyl-5-(3',4',5'-trimethoxyphenyl)pyrrole ZLM-12, 181 mg white solid was obtained, yield 66% , mp 168.1-170.2℃. 1 H NMR (400MHz, CDCl 3 ): δ3.77(s, 6H), 3.81(s, 3H), 3.86(s, 3H), 6.50(s, 2H), 6.75(d, J=8.4Hz, 1H), 6.78(d, J= 2.4Hz, 1H), 6.83 (dd, J=6.4, 2.0Hz, 1H). 13 C NMR (400MHz, CDCl3): δ55.5, 56.0, 60.9, 106.4, 110.7, 118.6, 122.9, 125.3, 132.6, 136.4, 136.8, 147.2, 153.3. MS (EI) m / z: 321 (M + ).
Embodiment 3
[0081] Preparation of 3,4,5-trimethoxy-1-[(4-nitrophenyl)thio]benzene (ZLM-8)
[0082]
[0083] According to Example 1, p-nitroiodobenzene (200 mg) was used instead of 3-nitro-4-methoxy iodobenzene to obtain 194 mg of light yellow solid, yield 76%, mp 168.1-170.2 ° C. 1 H NMR (400MHz, CDCl 3 ): δ3.85(s, 6H), 3.91(s, 3H), 6.79(s, 2H), 7.17(d, J=8.8Hz, 2H), 8.09(d, J=8.8Hz, 2H). 13C NMR (400MHz, CDCl3): δ56.3, 61.0, 112.0, 124.1, 124.2, 125.9, 138.4, 145.1, 149.1, 154.1. MS (EI) m / z: 321 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com