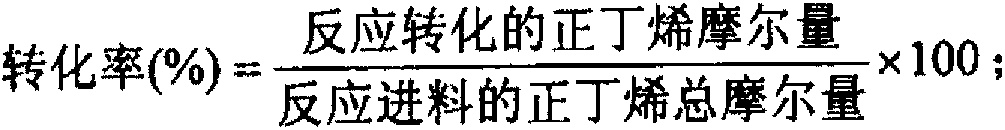Iron catalyst used in butadiene production through n-butylene oxidation dehydrogenation, and preparation method and application thereof
An oxidative dehydrogenation, iron catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. The problem of obvious effect, etc., achieves the effects of good reaction stability, high butadiene selectivity, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
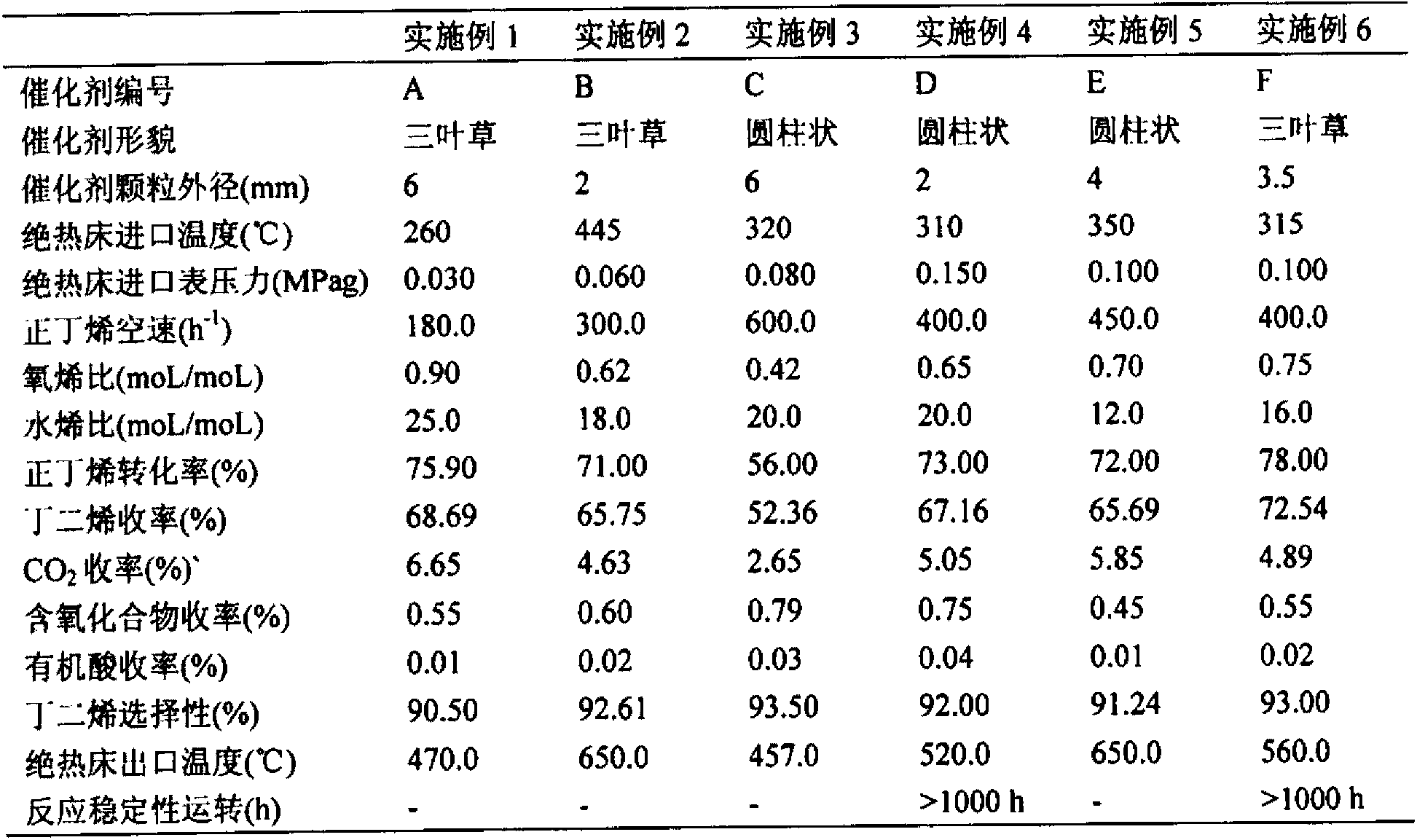
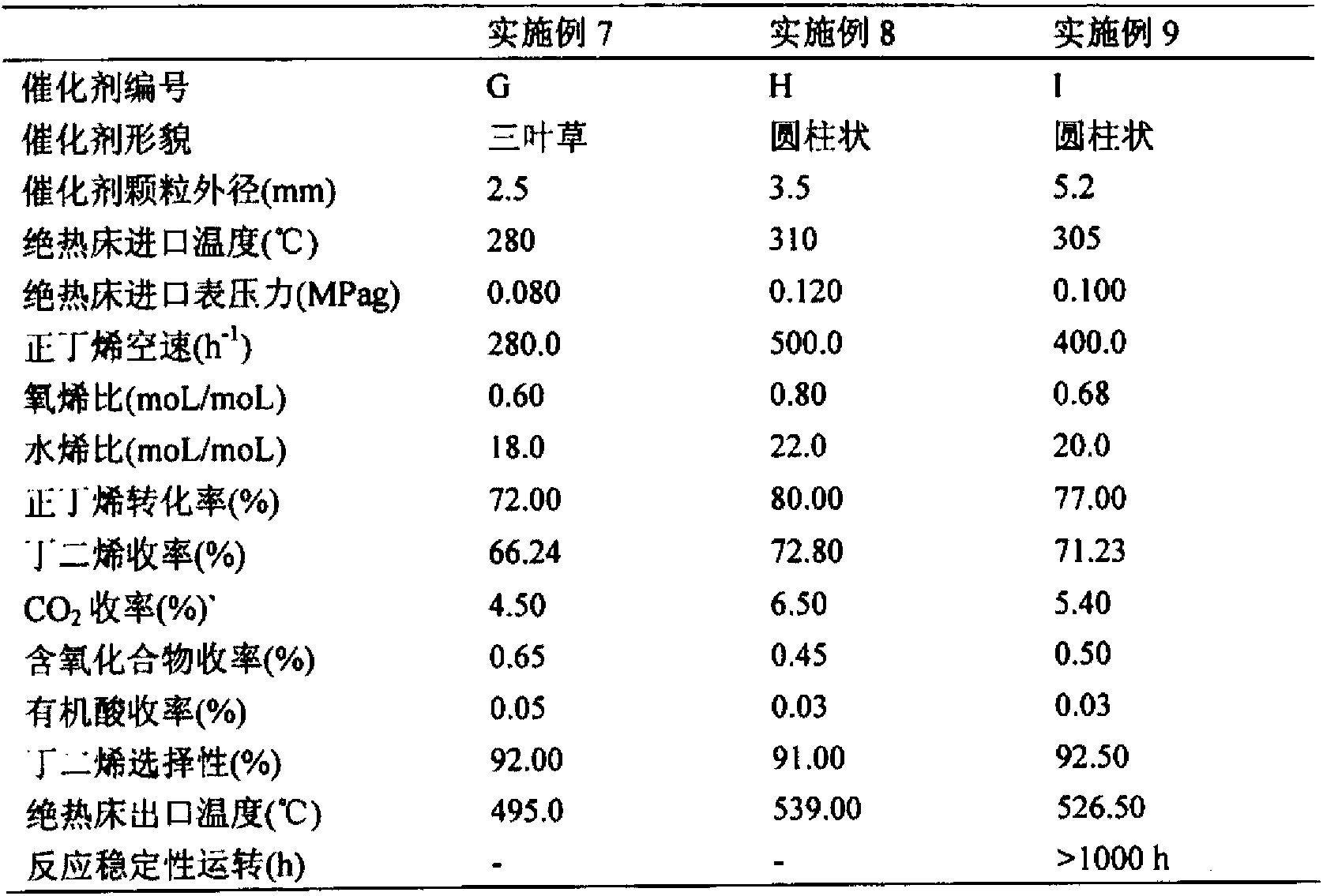
[0027] After the iron powder and metal zinc are dissolved in nitric acid solution, magnesium nitrate and manganese nitrate are added, and the final configuration is 4.43×10 -3 Mole of magnesium (Mg) / one mole of iron (Fe), 0.247 mole of zinc (Zn) / one mole of iron (Fe) and 0.109 mole of manganese (Mn) / one mole of iron (Fe) concentration, and then mix Add ammonia water dropwise to the metal salt solution, stir well, control the temperature of the precipitation slurry at 60.0°C, control the pH value of the precipitation end point at 7.50, maintain the pH value after the precipitation end point, continue stirring and aging for 60 minutes, filter the precipitation slurry and wash until pH = 7.0-9.0, Then the filter cake is extruded, dried at 200.0°C for 12h, and then activated at 500.0°C for 48h. The prepared catalyst is numbered A, and its mass composition is: iron (Fe) 51.85wt%, magnesium (Mg) 0.01wt%, manganese (Mn) 5.00wt%, zinc (Zn) 15.00wt%, and the rest is oxygen (O). The adi...
Embodiment 2
[0029] The steel is dissolved in a mixed solution of nitric acid and hydrochloric acid, then mixed with ferric nitrate nonahydrate, magnesium nitrate hexahydrate, cobalt nitrate and water and stirred evenly, and finally configured into 0.846 moles of magnesium (Mg) / one mole of iron (Fe), 3.88× 10 -3 Mole of cobalt (Co) / one mole of iron (Fe) concentration mixed metal salt solution, then the mixed metal salt solution is added dropwise in ammonia water, fully stirred, the temperature of the precipitation slurry is controlled at 60.0 ° C, the pH value of the precipitation end point is controlled at 11.0, and then Immediately filter the precipitated slurry and wash it to pH = 7.0-9.0, dry the filter cake at 105.0°C for 12h, then slice it into a granular catalyst, and activate it at 700.0°C for 16h. The prepared catalyst is numbered B, and its mass composition is: iron (Fe) 48.87wt%, magnesium (Mg) 18.00wt%, cobalt (Co) 0.20wt%, and the rest is oxygen (O). The adiabatic fixed bed re...
Embodiment 3
[0031] The steel is dissolved in a mixed solution of nitric acid and hydrochloric acid, then mixed with magnesium nitrate hexahydrate, calcium nitrate, ammonium molybdate, and antimony chloride and stirred evenly. , 1.28×10 -2 One mole of calcium (Ca) / one mole of iron (Fe), 2.32×10 -3 One mole of molybdenum (Mo) / one mole of iron (Fe), 2.96×10 -3 Mixed metal salt solution with molar antimony (Sb) / one molar iron (Fe) concentration, then add ammonia water to the mixed metal salt solution dropwise, stir well, control the temperature of the precipitation slurry at 80.0°C, control the pH value of the precipitation end point at 8.30, and control the precipitation end point Then maintain the pH value, continue to stir and age for 30 minutes, filter the precipitated slurry and wash to pH = 7.0-9.0, dry the filter cake at 200.0°C for 5 hours, and then punch it into a granular catalyst, and activate it at 850.0°C for 4 hours, the prepared catalyst The code is C, and the mass compositio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com