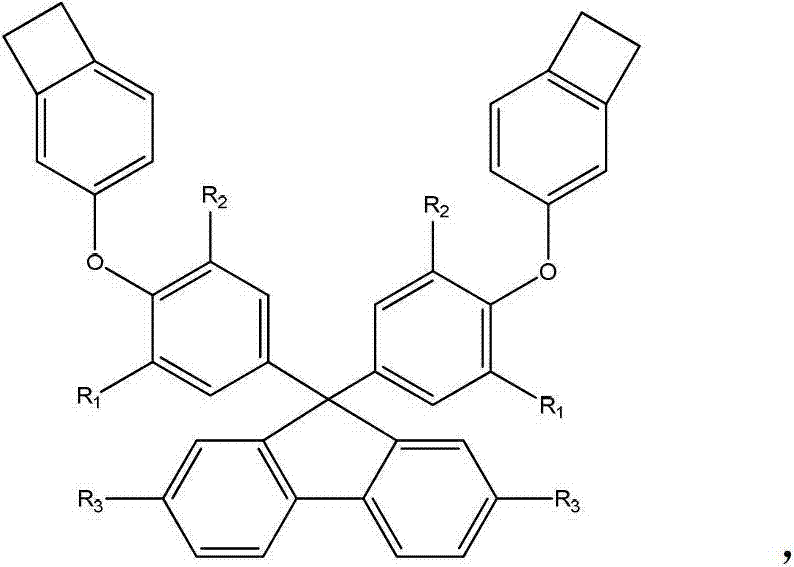
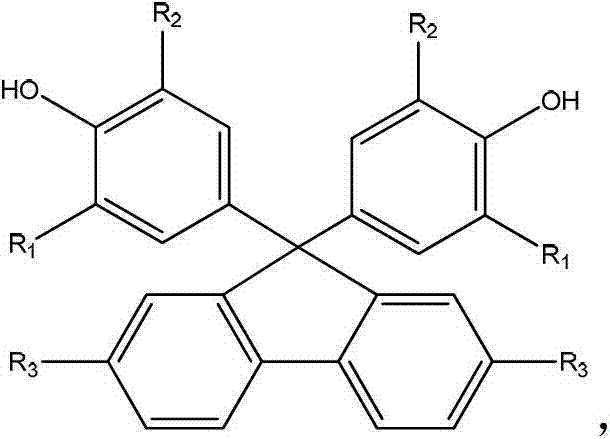
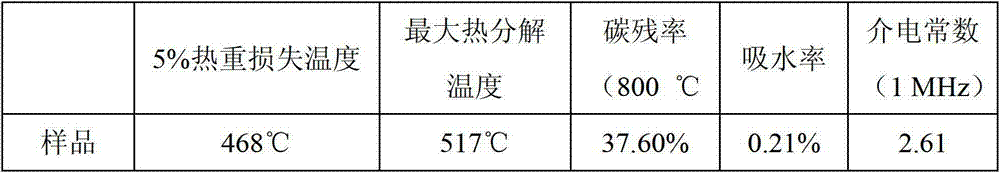
Thermosetting resin monomer containing fluorene and benzocyclobutene construction unit as well as preparation method and application thereof
A technology of benzocyclobutene and bromobenzocyclobutene, which is applied in the field of high-performance polymer manufacturing, can solve the problems of unstable heat resistance and dielectric properties of films, incomplete progress and the like, and achieves low moisture absorption, The effect of good temperature resistance and high chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0024] Synthesis of embodiment 19,9-bis(3-fluoro-4-hydroxyphenyl)fluorene
[0025] Under the protection of nitrogen, 36.2 grams of fluorenone, 90.1 grams of o-fluorophenol, 0.3 milliliters of β-mercaptopropionic acid and 100 milliliters of toluene were added to the reaction device, and after stirring for 0.5 hours at 30 ° C, 5 milliliters of concentrated After the addition of sulfuric acid, the temperature was raised to 55°C to react for 5 hours, cooled to room temperature, the product was poured into water, solids were precipitated, and recrystallized in toluene to obtain white crystals of fluorine-containing bisphenolfluorene with a yield of 92%. Melting point, 220-221°C (literature, USP5304688, 219-221°C). Mass spectrometry (EI-MS) characterized m / z: 386.11 (100.0%), 387.12 (27.3%), 388.12 (4.0%).
Embodiment 29
[0026] Synthesis of embodiment 29,9-bis(3-trifluoromethyl-4-hydroxyphenyl)fluorene
[0027] Under nitrogen protection, 18.2 grams of fluorenone, 64.8 grams of o-trifluoromethylphenol, 0.4 milliliters of β-mercaptopropionic acid and 80 milliliters of toluene were added to the reaction device, and after stirring for 0.5 hours at 30°C, slowly dropwise added 4 milliliters of concentrated sulfuric acid, after the dropwise addition, the temperature was raised to 55°C for 6 hours, cooled to room temperature, the product was neutralized with saturated sodium bicarbonate solution, washed with water until neutral, and the organic phase was rotary evaporated to obtain a solid crude product, which was passed through column chromatography ( Petroleum ether / ethyl acetate, 4:1) to obtain white crystals of fluorine-containing bisphenolfluorene with a yield of 85%. Mass spectrometry (EI-MS) characterization m / z: 486.11(100.0%), 487.11(29.5%), 488.11(4.5%)
Embodiment 32
[0028] Synthesis of Example 32,7-bis(trifluoromethyl)-9,9-bis(4-hydroxyphenyl)fluorene
[0029] Under the protection of argon, add 8.2 g of 2,7-dibromo-9,9-bis(4-hydroxyphenyl)fluorene to the reaction device (prepared according to the literature: R. Grisorio et al., Macromolecules 2011, 44, 7977-7986) , 2 grams of three (dibenzylideneacetone) dipalladium (i.e. Pd 2 (dba) 3 ), 4.23 grams of cuprous iodide, 32 milliliters of methyl fluorosulfonyl difluoroacetate and 100 milliliters of N,N-dimethylformamide, reacted at 100 ° C for 12 hours, cooled to room temperature, and the product was poured into water, with A solid precipitated out and was recrystallized in toluene to obtain 2,7-bis(trifluoromethyl)9,9-bis(4-hydroxyphenyl)fluorene as white crystals with a yield of 86%. Mass spectrometry (EI-MS) characterization m / z: 486.11(100.0%), 487.11(29.5%), 488.11(4.5%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com