Rosin-based chain extender and preparation method thereof
A technology based on chain extender and rosin, which is applied in the field of rosin-based chain extender and its preparation to achieve high recovery effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Rosin diacid (formula III, R 1 benzene ring segment) 51.926g, ethylene glycol (HO-R 2 -OH,R 2 for C 2 aliphatic segment) 12.4g, sulfuric acid 0.04g, reacted at 150°C for 5h under the protection of nitrogen, washed with 0.1mol NaOH aqueous solution after the reaction, removed excess ethylene glycol and possibly unreacted rosin diacid to obtain crude product, The crude product was washed with methanol and dried under vacuum to obtain a rosin-based chain extender with the structure of formula I (ie, ethylene glycol rosin dioate).
[0058] Among them, R 1 for
[0059] Formula III
[0060] Among them, R 1 for
[0061] Formula IR 2 for
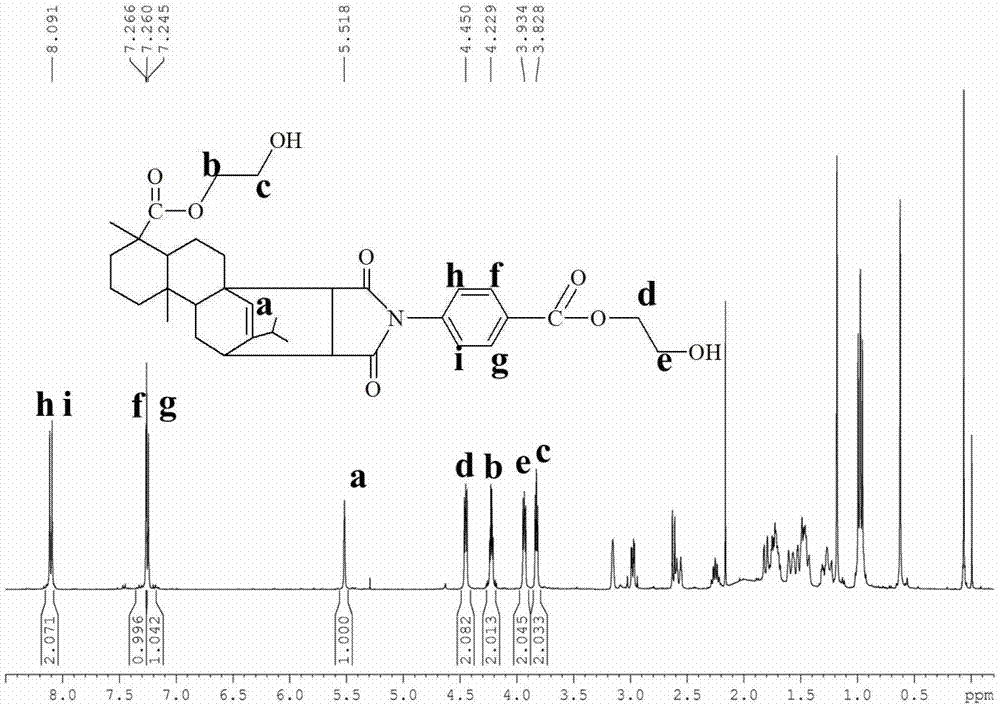
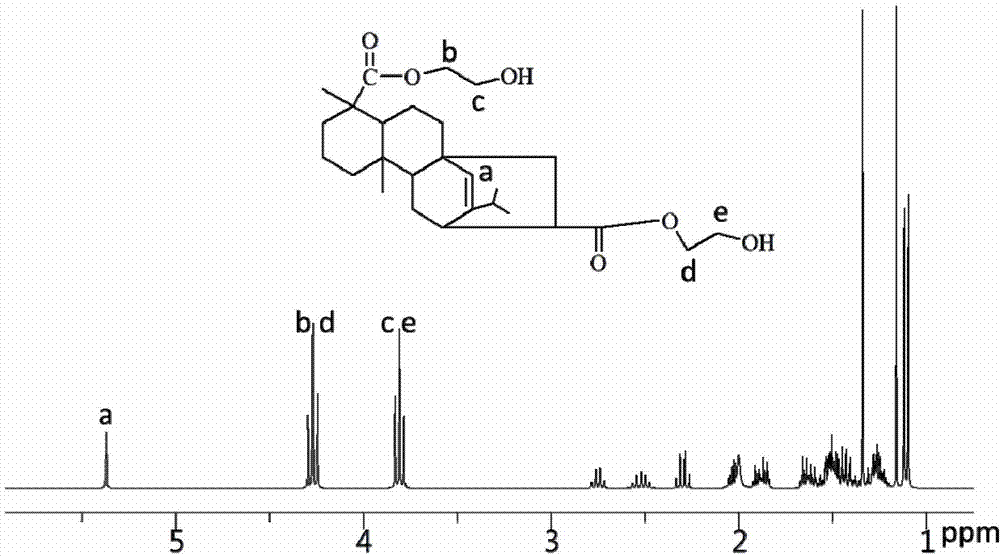
[0062] The NMR spectrum of the rosin-based chain extender of the formula I structure prepared in Example 1 is as figure 1 As shown, the solvent is deuterated chloroform, and the internal standard is tetramethylsilane. δ=5.5ppm is the hydrogen on the double bond on the rosin skeleton (shown at position a on the figure), an...
Embodiment 2
[0065] Rosin diacid (formula IV) 37.4g, ethylene glycol (HO-R 2 -OH,R 2 for C 2 aliphatic segment) 12.4g, p-toluenesulfonic acid 0.04g, reacted at 150°C for 5h under nitrogen protection, washed with 0.1mol NaOH aqueous solution after the reaction, removed excess ethylene glycol and possibly unreacted abietic diacid to obtain crude product, The crude product was washed with ethanol, and dried in vacuum to obtain a rosin-based chain extender with a structure of formula II (ie, ethylene glycol rosin dioate).
[0066]
[0067] Formula IV Formula II
[0068] Among them, R 2 for
[0069] The NMR spectrum of the rosin-based chain extender of the formula II structure prepared in Example 2 is as follows figure 2 As shown, the solvent is deuterated chloroform, and the internal standard is tetramethylsilane. δ=5.5ppm is the hydrogen on the double bond on the rosin skeleton (shown at position a on the figure), and its integral area is 1, which can prove the existence of the ro...
Embodiment 3
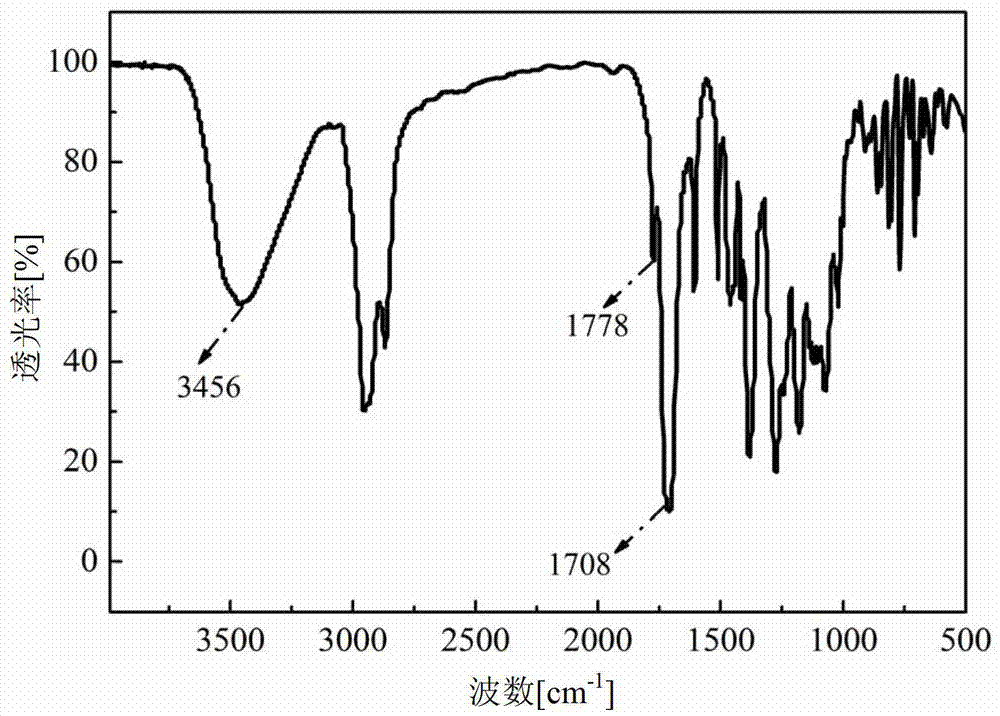
[0071] Rosin diacid (formula III, R 1 Methylene) 45.73g, methanol 6.4g, phosphoric acid 0.036g, react at 45°C for 5h, distill off excess methanol under reduced pressure to obtain dimethyl abietate. Then add 36g of 1,3-propanediol (HO-R 2 -OH,R 2 for C 3 The aliphatic chain segment) was reacted at 150°C, methanol was distilled off during the reaction, and the reaction ended after 5 hours. After the reaction, wash with water to wash off excess 1,3-propanediol to obtain a crude product, then wash with methanol, and dry in vacuum to obtain a rosin-based chain extender with the structure of formula I (ie propylene glycol rosindialate). It can be proved that the prepared rosin-based chain extender has the structure of formula I by proton nuclear magnetic spectrum and infrared spectrum.
[0072] Among them, R 1 for
[0073] Formula III
[0074] Among them, R 1 for
[0075] Formula IR 2 for
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com