Nanoparticle-loaded microsphere system for injection and preparation method of system
A nanoparticle and injection technology, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of easy aggregation, instability, and poor sustained release of nanoparticles. To achieve the effect of enhanced sustained release, enhancement and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
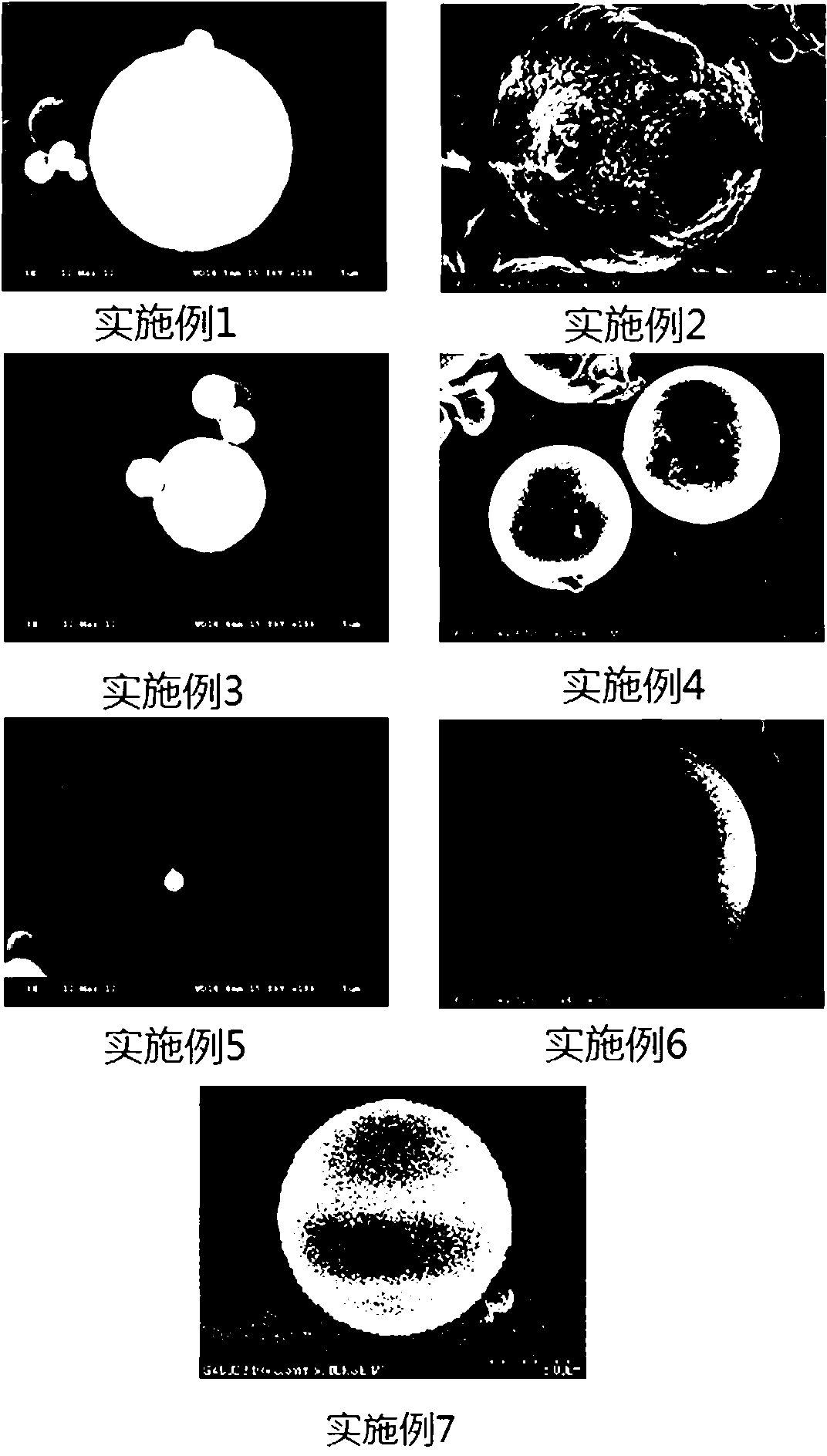
Image
Examples
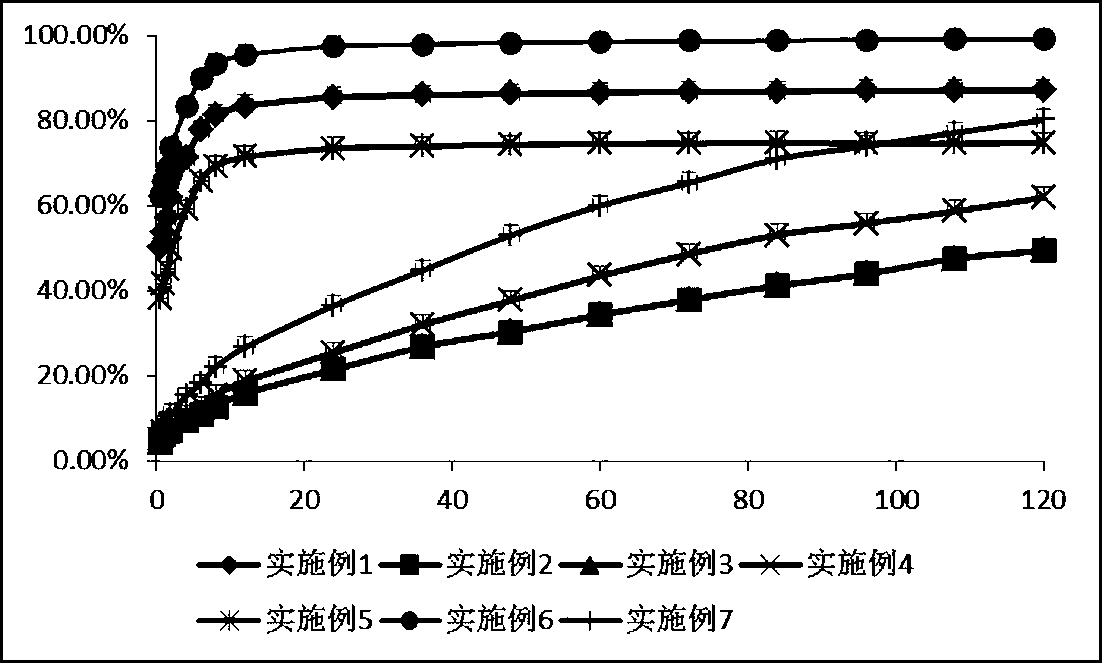
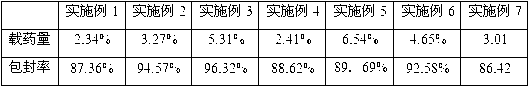
Embodiment 1
[0044] Dissolve 100mg of BSA in 2ml of deionized water, adjust the pH to 9 with 0.1mol / l NaOH; dissolve 20mg of strychnine in 3ml of absolute ethanol, and add strychnine dropwise into the albumin solution while stirring rapidly After dispersing for 30 minutes, add 2ml of absolute ethanol solution dropwise for dehydration, add 1000ul of 0.25% glutaraldehyde aqueous solution, continue to stir and solidify for 10 hours, and remove the organic solvent by rotary evaporation at 35°C (35-40°C) . lyophilized. To prepare a 1% (v / v) acetic acid solution, dissolve CS in 0.5% (w / v) acetic acid. Brucine-BSA-Nps was added into the CS acetic acid solution at a ratio of 1:1 (BSA:CS) while stirring. Disperse for 1 hour, add 1% (v / v) glutaraldehyde (CS: glutaraldehyde) at a ratio of 10:1 while stirring, and cure for 1 hour. Spray dry. The air pressure is 40ml / h, the inlet temperature is 120°C, and the speed of the peristaltic pump is 20%.
Embodiment 2
[0046] Dissolve 100mg of BSA in 2ml of deionized water, and adjust the pH to 9 with 0.1mol / l NaOH; dissolve 20mg of cortisone in 3ml of absolute ethanol, and add nuxonum dropwise to the albumin solution while stirring rapidly Alkaline absolute ethanol solution, after dispersing for 30 minutes, continue to drop 2ml of absolute ethanol solution for dehydration, add 1000ul 0.25% glutaraldehyde aqueous solution, continue to stir and solidify for 10 hours, and remove the organic solvent by rotary evaporation at 35 ℃ (35-40). lyophilized. Weigh 36 mg of nanoparticles and dissolve in 200 μL of water as the inner water phase; in addition, take PLGA according to the ratio of excipients 1:1 (PLA:PGA=50:50, M r =6000) was dissolved in 4mL of dichloromethane as the oil phase, the probe was ultrasonicated, and after fully emulsified, 1% PVA solution was added at a ratio of 1:1 (PLGA:PVA), emulsified at a speed of 1000r / min for 3min, and the magnet was stirred at 500rpm for 1h. After stand...
Embodiment 3
[0048] Weigh 36 mg of hydrocortisone and dissolve it in 200 μL of water as the inner water phase; take another PLGA (PLA:PGA=50:50, M r =6000) 330mg dissolved in 4mL of dichloromethane as the oil phase, ultrasonic probe, after fully emulsified, add 1% PVA solution at 1:2 (PLGA:PVA), emulsify at 9000r / min for 3min, and stir at 500rpm for 1h , After standing still until the air bubbles in the table machine basically disappear, freeze-dry. To prepare a 1% (v / v) acetic acid solution, dissolve CS in 0.5% (w / v) acetic acid. The nanoparticles were added into the CS acetic acid solution with stirring at a ratio of 1:10 (PLGA:CS). Disperse for 1 hour, add 1% (v / v) glutaraldehyde at a ratio of 1:1 (CS: glutaraldehyde) while stirring, and cure for 1 hour. Spray dry. The air pressure is 40ml / h, the inlet temperature is 120°C, and the speed of the peristaltic pump is 20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com