Efficient secretory expression and purification method of recombinant urate oxygen oxidoreductase
A urate oxidase and urate oxidase protein technology, applied in the field of medical bioengineering, can solve the problems of poor cytoplasmic expression, difficult control of protease cleavage conditions, non-specific cleavage, etc., and achieve low product cost and high purification The effect of short cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
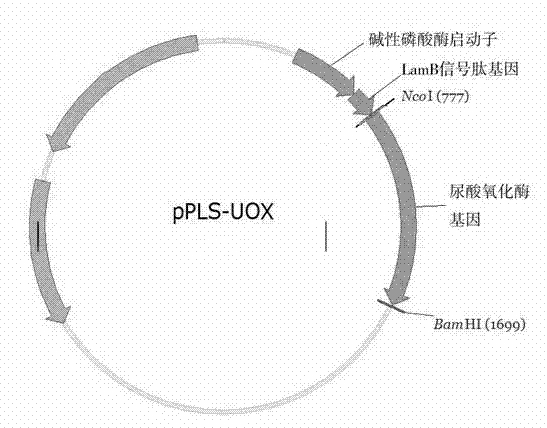
[0021] Example 1 Construction of recombinant urate oxidase secretion expression plasmid pPLS-UOX (see figure 1 ).
[0022] 1. Design and synthesize the Escherichia coli alkaline phosphatase promoter (phoA promoter).
[0023] EcoRI and XbaI restriction enzyme cutting sites were introduced at the 5' end and 3' end of the sequence of the Escherichia coli alkaline phosphatase promoter (phoA promoter), respectively. The designed and synthesized oligonucleotide sequence is SEQ1.
[0024] 2. Design and synthesize the coding sequence of Escherichia coli LamB signal peptide (LamB signal peptide) plus recombinant urate oxidase.
[0025] The coding sequence of the natural Escherichia coli LamB signal peptide is:
[0026] ATGATGATTA CTCTGCGCAA ACTgCCTCTG GCGGTTGCCG TCGCAGCGGG CGTAATGTCT GCTCAGGCAA TGGCTGTTGA TTTC
[0027] According to the codon preference of Escherichia coli, taking into account the influence of the secondary structure of post-transcriptional mRNA and the hydrophobici...
Embodiment 2
[0032] Example 2 Construction of engineering strain PLS-UOX for secretion and expression of recombinant urate oxidase.
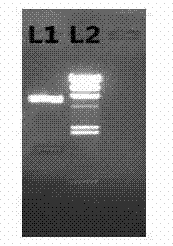
[0033] E. coli empty host W3110 was transformed with plasmid pPLS-UOX, and the transformants were inoculated in AP5 medium and cultured in shake flasks at 37°C. The expression results were identified according to SDS-PAGE electrophoresis ( image 3 ) preferably obtains engineering bacteria that secrete and express recombinant urate oxidase.
[0034] The composition of AP5 medium is:
[0035] 0.15% glucose
[0036] 1.1% Acid Hydrolyzed Casein
[0037] 0.06% yeast extract
[0038] 0.019% MgSO 4
[0039] 0.107% NH 4 Cl
[0040] 0.373% KCl
[0041] 0.12% NaCl
[0042] 0.12mol / L triethanolamine pH7.4.
Embodiment 3
[0043] Example 3 Separation and Purification of Recombinant Urate Oxidase
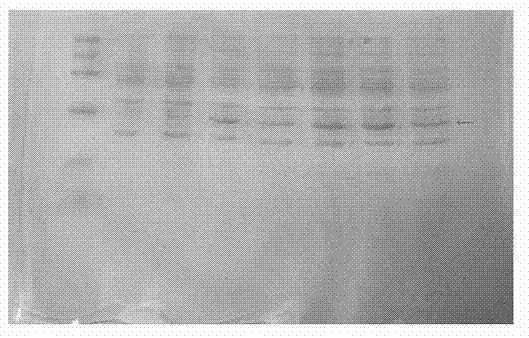
[0044] Recombinant urate oxidase cells are crushed by high-pressure homogenization, the pressure is 30-40MPa, the centrifugal force of the broken bacteria is not less than 8000g, and the centrifuged supernatant is collected; the Cond value of the supernatant protein liquid is lower than the Cond value of the cationic chromatography equilibrium liquid Carry out cation chromatography, collect the sample, and the electrophoresis identification result of the purification effect is as follows: Figure 4 As shown; the pH value of the sample for cationic chromatography is adjusted to be consistent with the pH value of the hydrophobic chromatography equilibrium solution, and the hydrophobic chromatography is carried out to collect the sample for the sample, and the purification effect electrophoresis identification result is as follows Figure 5 Shown; The sample Cond value of the hydrophobic chromatography is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com