Specific inhibition compound of SAHN enzyme protein and synthetic method of specific inhibition compound
A compound and specific technology, applied in the direction of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve the problem of ineffective antibiotic treatment, and achieve the effect of good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Cloning, expression and purification of Escherichia coli SAHN, SRHH proteins and host SAHH proteins
[0035] 1. Construction of Escherichia coli gene sequence SAHN and SRHH recombinant vectors: obtain the corresponding gene sequences from Genebank, design PCR primers with Primer5.0, and use PCR amplification technology to obtain DNA sequences from E.coli XL1-Blue respectively . The PCR product was connected to the previously constructed pYEMF-T vector, transformed into E.coli K-12DH5α, selected white positive clones, extracted the plasmid after cultivation, separated and identified by agarose electrophoresis after enzyme digestion, and the digested fragments were passed through the MagExtractor (TOYOBO Inc.) Kit Purified, cloned into pCA24N plasmid, transformed into competent cells BL21 (DE3), and screened on chloramphenicol or ampicillin LB plates.
[0036] 2. Construction of human gene sequence SAHH recombinant vector: Human placenta tissue was quick-frozen in liquid...
Embodiment 2
[0039] Establishment of Enzyme Coupled Assay Detection System for SAHN and SAHH
[0040] S-adenosyl homocysteine nuclease (SAHN) enzyme coupling assay detection reaction mechanism is as follows:
[0041]
[0042] The analysis and detection reaction mechanism of S-adenosylhomocysteine hydrolase (SAHH) is as follows:
[0043]
[0044] The enzyme coupling analysis detection system contains 0.1-20μM different concentrations of SAH, 10μM SRHH (SRHH is not added for SAHH enzyme detection), 1.0mM dithiothreitol, 50mM Hepes buffer at pH 7.4 and different concentrations of enzyme inhibitors After shaking and balancing in a water bath at 37°C for 5 minutes, add SAHN or SAHH protein to start the enzyme reaction, react at 37°C for 10 minutes, and use four times the volume of 5,5'-dithio-2,2'-bisnitrobenzoic acid ( DTNB) (133μM)-guanidine hydrochloride (8M) solution to terminate the reaction, 412nm colorimetric measurement of the generated 5-thio-2-nitrobenzoic acid (TNB), and d...
Embodiment 3
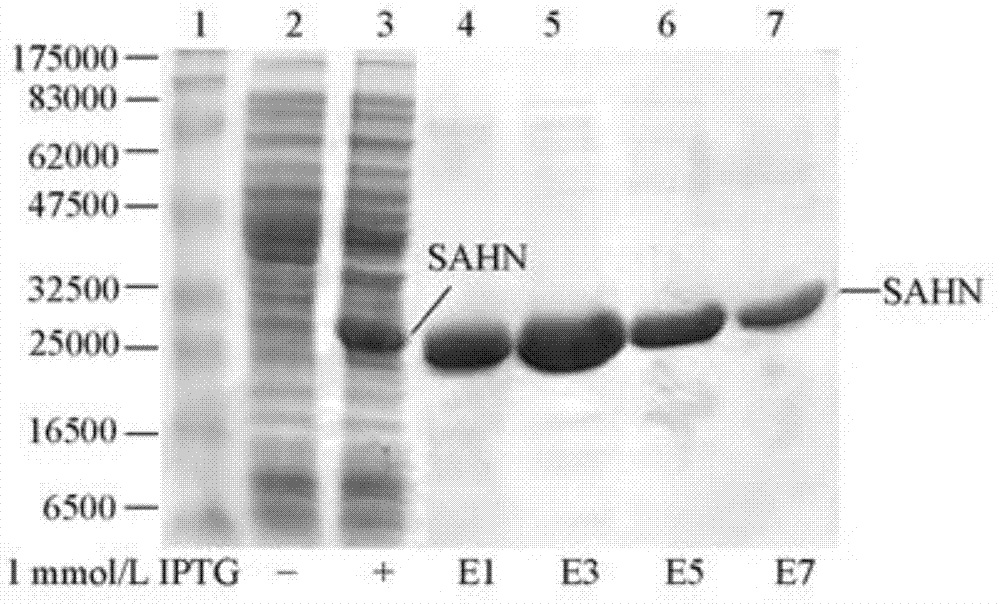
[0046] 1. The SAHN sequence was obtained by PCR amplification, and the pEX-sahn expression vector was constructed. After transforming BL21(DE3), IPTG induced expression to obtain the SAHN fusion protein with a histidine tag. Ni 2+ Agarose affinity chromatography column adsorption, imidazole elution, separation and purification to obtain recombinant enzyme protein, as shown in the attached figure figure 1 shown.
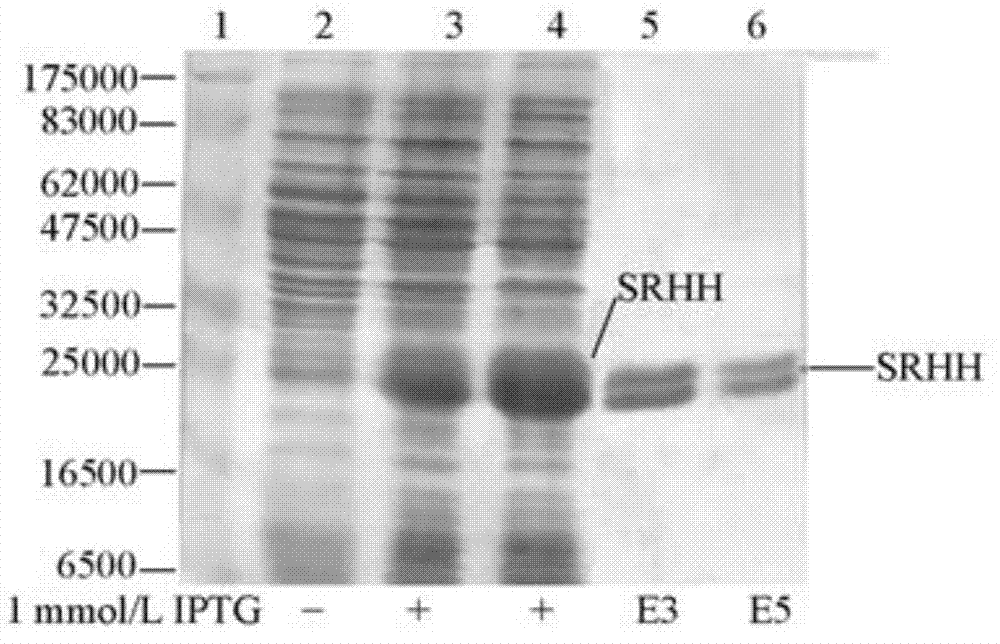
[0047] 2. Construct pCA-SRHH expression vector, after transforming BL21(DE3), induce expression with IPTG to obtain SRHH fusion protein with histidine tag, absorb on Ni2+ agarose affinity chromatography column, elute with imidazole, separate and purify to obtain recombinant enzyme protein SRHH, the protein can be used for enzyme coupling assay detection of SAHN enzyme activity, as shown in the accompanying drawing figure 2 shown.
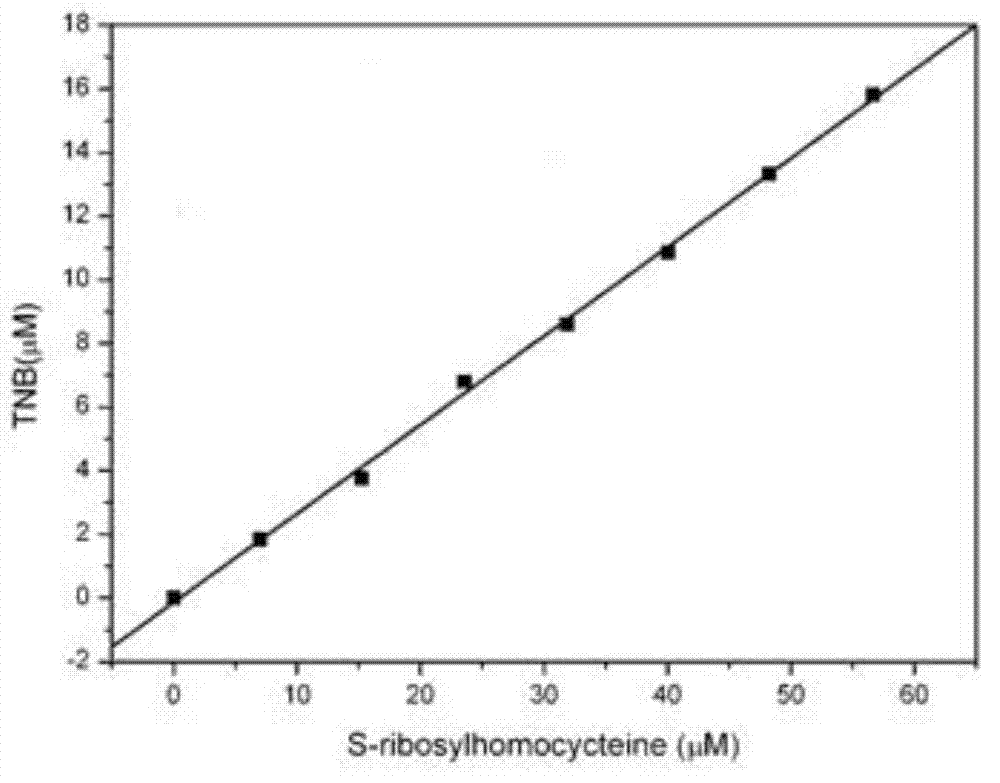
[0048] 3. Linear correlation analysis of SAHN nucleosidase activity detected by enzyme coupling analysis:
[0049] Based on the enzyme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com