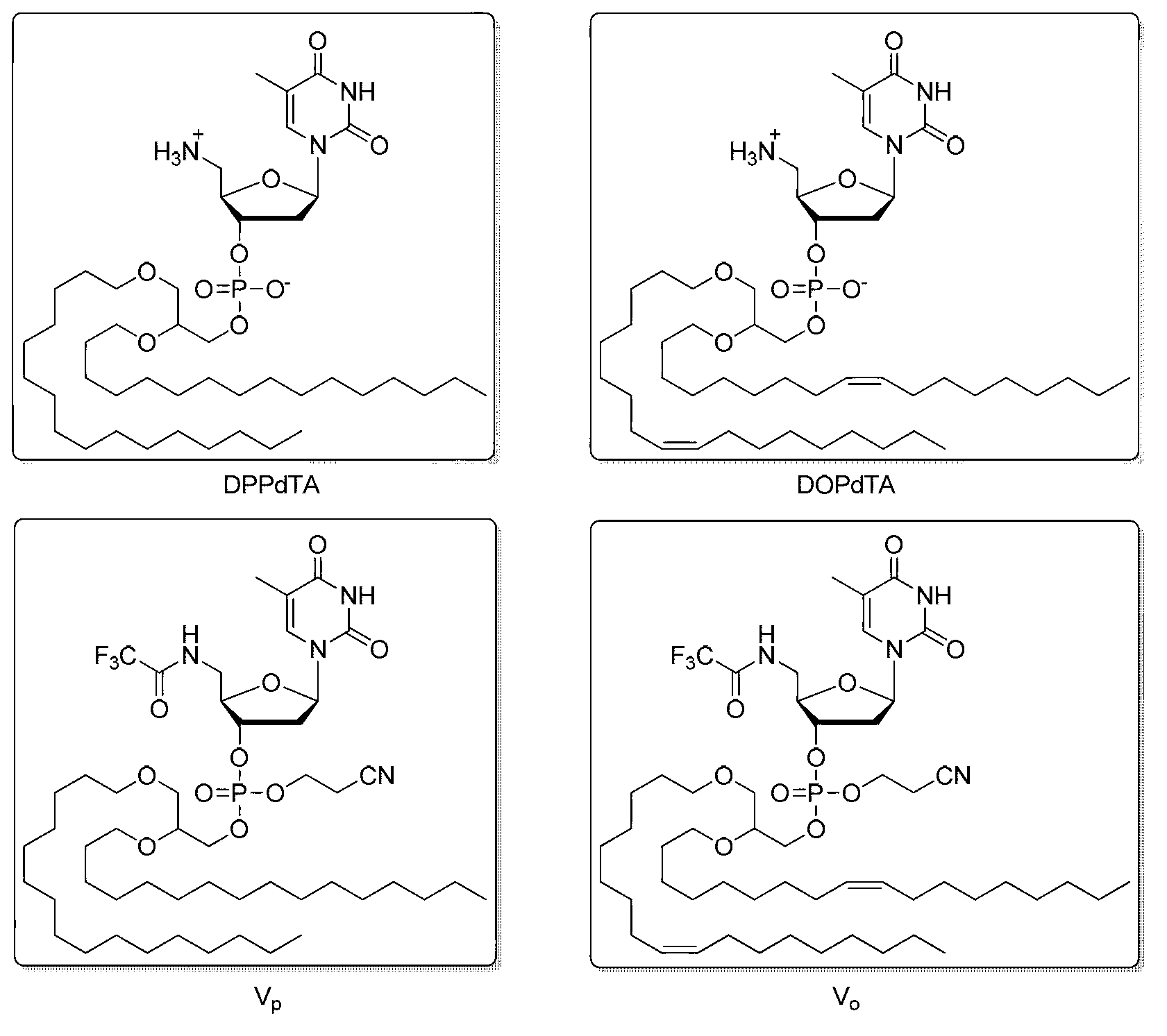
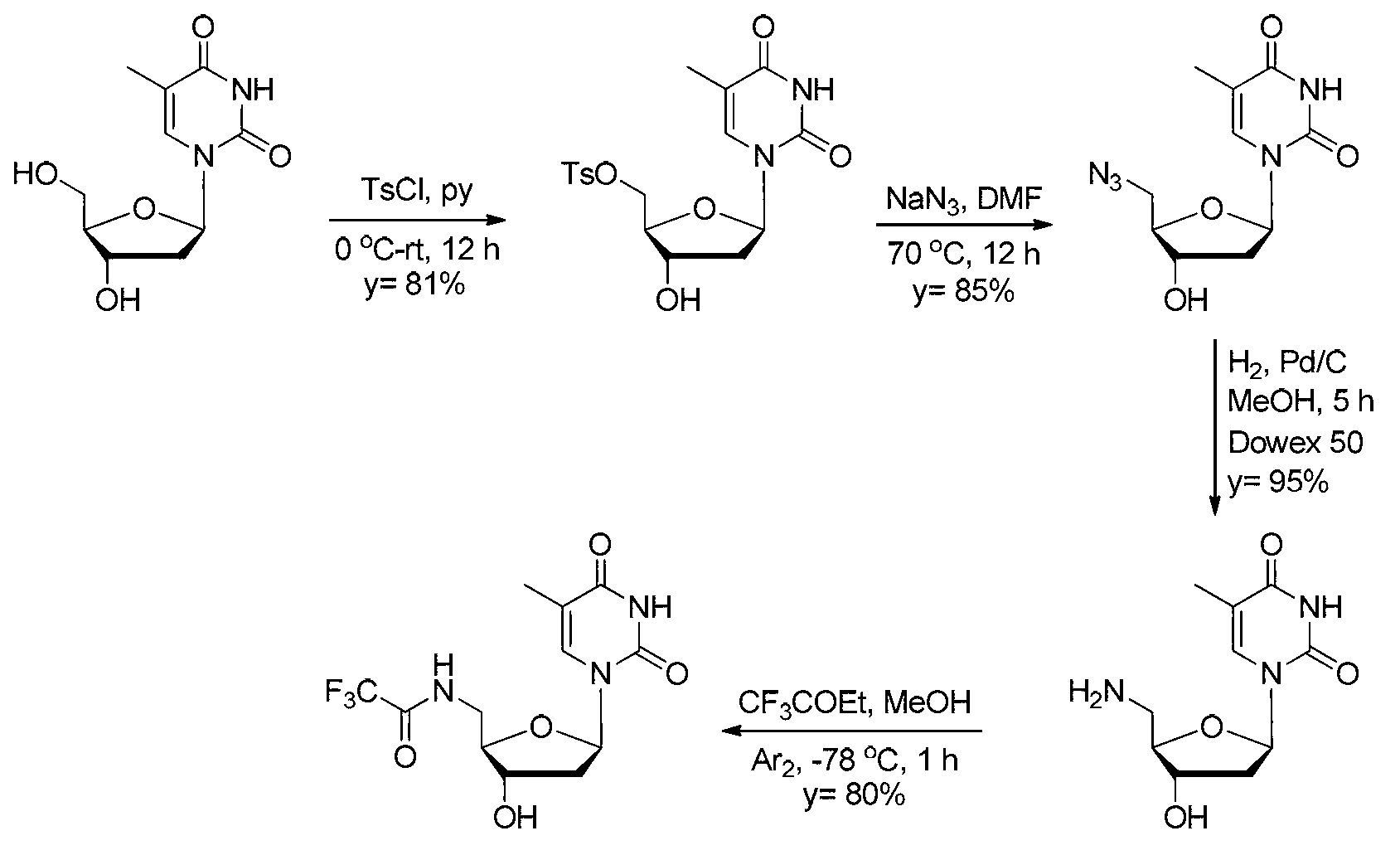
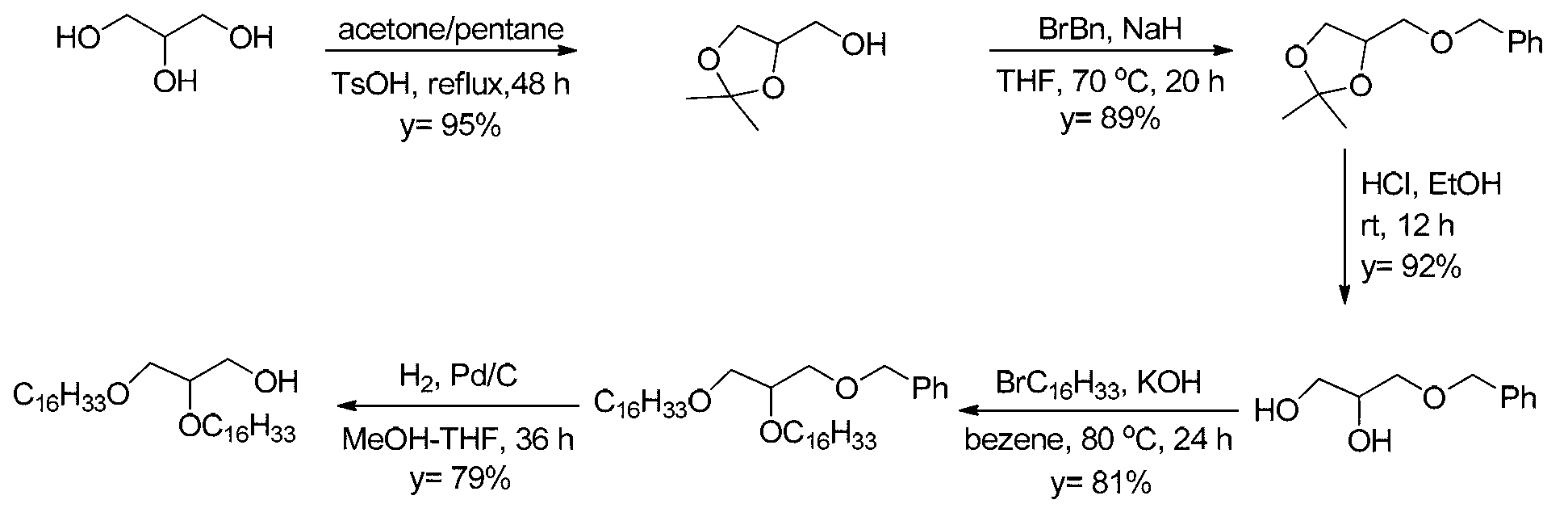
5'-amino-2',5'-dideoxynucleotide phospholipid molecules, and preparation method and application thereof
A technology of deoxynucleotide phospholipids and deoxynucleoside phospholipids, which is applied in the field of new biomaterials, can solve the problems of difficult effective release, high cytotoxicity and serum toxicity of cationic liposomes, and achieves good efficacy, simple and efficient synthesis method, and preparation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] [Example 1] Synthesis of 5'-p-methylbenzenesulfonyl thymidine
[0067] Thymidine (24.2 g, 0.1 mol) was dissolved in anhydrous pyridine (200 mL), and the temperature was cooled to 0 °C in an ice bath. p-Toluenesulfonyl chloride (23 g, 0.12 mol) was dissolved in anhydrous pyridine (100 mL), and slowly added dropwise to the aforementioned reaction solution through a micro injector, and the dropwise addition process continued for 6 h. The reaction process was always carried out at 0 °C under the protection of argon. After the dropwise addition, the reaction was returned to room temperature, and stirring was continued for 6 h. Pyridine was evaporated under reduced pressure. To the residue was added 500 mL of ethyl acetate and NaHCO 3 Aqueous solution (10%) 300 mL, a large amount of white solid was precipitated. Filtration, the obtained white solid is the target product. The filtrate was collected, the organic phase was separated, and then the aqueous phase was washed wi...
Embodiment 2
[0068] [Example 2] Synthesis of 5'-azido-2',5'-dideoxythymidine
[0069] 5'-p-methylbenzyl thymidine (5 g, 12.6 mmol) was dissolved in dry DMF (25 mL), to which was added NaN 3 (1.2g, 19mmol), heated to 70°C, and reacted under argon protection for 12h. The solvent was evaporated under reduced pressure. To the residue was added dichloromethane (50 mL), followed by washing with water (30 mL). Anhydrous Na for organic phase 2 SO 4Dry and separate by silica gel column chromatography, eluent DCM:MeOH=20:1, to obtain 2.9 g of the target product (yield 85%). white solid. 1 H NMR (400MHz, DMSO-d 6 ):δ=11.31(s,1H),7.49(s,1H),6.20(t,J=7.2Hz,1H),5.39(d,J=4.0Hz,1H),4.20(s,1H),3.79 -3.85(m,1H),3.56(d,J=5.2Hz,2H),2.20-2.30(m,1H),2.05-2.15(m,1H),1.79(s,3H); 13 C NMR (100MHz, DMSO-d 6 ):δ=163.7, 150.5, 136.1, 109.8, 84.6, 83.9, 70.7, 51.6, 38.1, 12.1; 1298.8, 1272.8, 1067.3, 963.4, 856.2, 636.4, 553.4, 493.8cm -1 ;MS(ESI-TOF+)for C 10 h 13 N 5 o 4 Na[M+Na] + found 290.1042,cal...
Embodiment 3
[0070] [Example 3] Synthesis of 5'-amino-2',5'-dideoxythymidine
[0071] Take 5'-azido-2',5'-dideoxythymidine (5.5g, 20mmol) and dissolve it in methanol (150mL), add 10% palladium carbon catalyst (0.55g), and place it in a hydrogenation apparatus hydrogenation. The pressure is 60Psi. After hydrogenation at room temperature for 5 h, the reaction was stopped. TLC detection found that the raw material had reacted completely. Suction filtration through celite under reduced pressure to remove the solid matter, and the obtained filtrate was evaporated to dryness to obtain 4.8 g of light yellow solid. The solid is insoluble in organic solvents such as methanol and ethyl acetate, and is very polar, and cannot be separated by ordinary silica gel column chromatography. The resulting solid was dissolved in water and purified by Dowex 50, a strongly acidic ion exchange resin, to obtain 4.7 g of the target product with a yield of 95%. white solid. 1 H NMR (400MHz, DMSO-d 6 ):δ=7.65(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com