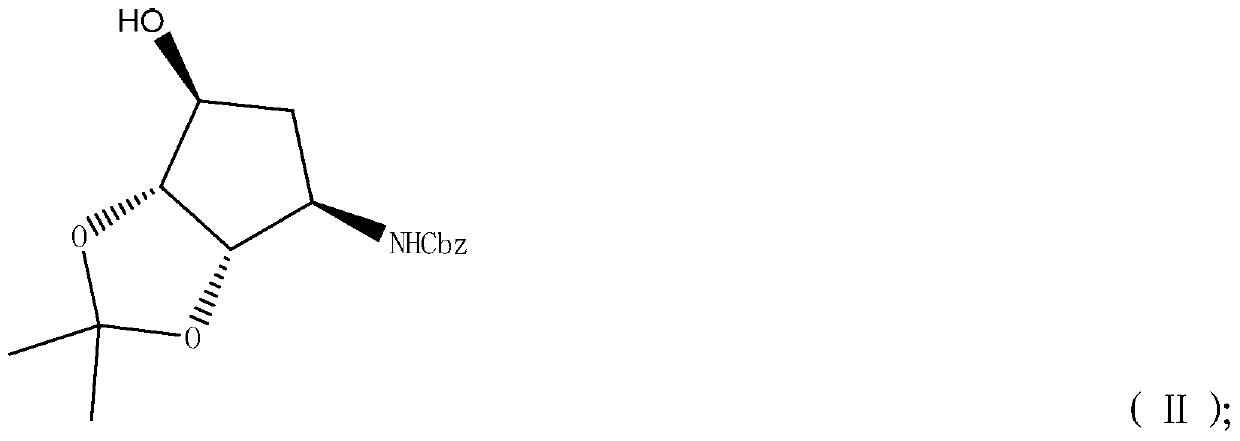Preparation method of ticagrelor midbody
A technology for ticagrelor and an intermediate, which is applied in the field of chemical synthesis, can solve the problems of being unsuitable for industrial production, high energy consumption for solvent recovery, and high equipment requirements, and achieves the effects of not easy to catch fire, low production cost, and good product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
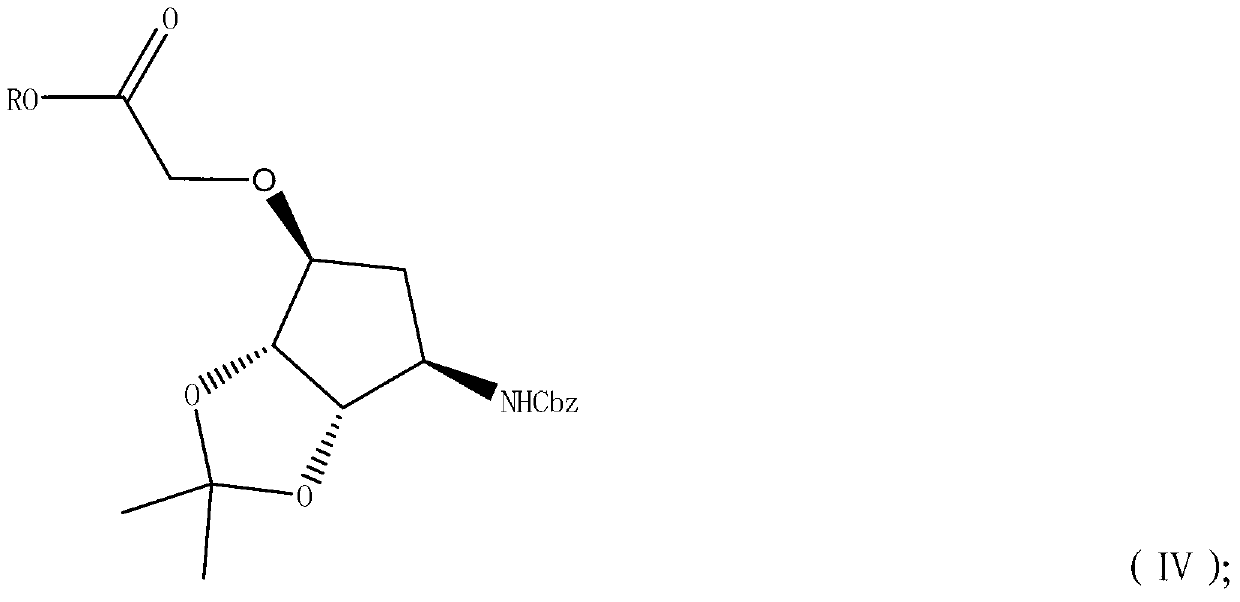
[0051]
[0052] Under nitrogen protection, 6.3g NaH (60% content) was suspended in 120mL tetrahydrofuran. 35.0 g of compound II was slowly added dropwise to the above suspension. 24.6 g of ethyl chloroacetate was added dropwise to the reaction solution, TLC detected that the reaction was complete, extracted with ethyl acetate, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate and concentrated to obtain 43 g of crude product of light yellow viscous liquid compound IV, The yield is 96% (based on compound I). 1 H NMR (500MHz, CDCl 3 )δ1.21-1.29 (m, 6H), 1.40 (s, 3H), 1.82 (d, J=14.5Hz, 1H), 2.18-2.23 (m, 1H), 3.91 (d, J=4.2Hz, 1H ),4.08(d,J=5.6Hz,1H),4.14-4.24(m,4H),4.57(s,2H),5.10(dd,J 1 =12.5Hz,J 2 =2.7Hz,2H),6.00(d,J=8.7Hz,1H),7.28-7.36(m,5H).
Embodiment 2
[0054]
[0055] 45g of compound IV was dissolved in tetrahydrofuran, 5g of sodium borohydride was added at room temperature, 10mL of methanol was slowly added dropwise to the above solution, and stirred overnight at room temperature. TLC showed that the reaction was complete, 150 mL of water was added, extracted with ethyl acetate, washed with saturated brine, the organic phase was dried over anhydrous sodium sulfate, concentrated, and the crude product was directly used for the next reaction.
Embodiment 3
[0057]
[0058] Dissolve 38.7g of compound V in ethanol, weigh 2.0g of 5% palladium carbon, and react in a hydrogen environment for 5 hours, and TLC detects that the reaction is complete. The palladium carbon was suction-filtered and concentrated, and the crude product was subjected to silica gel column chromatography to obtain 22.8 g of ticagrelor intermediate I, with a yield of 95%, an HPLC purity of over 98%, and a retention time consistent with that of the standard product. 1 H NMR (500MHz, CDCl 3 )δ1.27(s,3H),1.39(s,3H),1.95(d,J=12.0Hz,2H),2.10-2.14(m,1H),3.43(d,J=6.0Hz,1H), 3.55-3.63(m,2H),3.65-3.70(m,2H),3.90(s,J=4.2Hz,1H),4.57(d,J=5.4Hz,1H),4.66(d,J=5.0Hz ,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com