Apolygus lucorum membrane-bound trehalase, its coding sequence, vector and strain of sequence, and application of vector or strain
A technology of trehalase and chlorophyll, which is applied in the field of agricultural science, can solve the problems of lack of recombinant trehalase and no research, and achieves the effects of wide suitable reaction temperature range, speeding up reaction speed and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Acquisition of ALTre-2 Gene
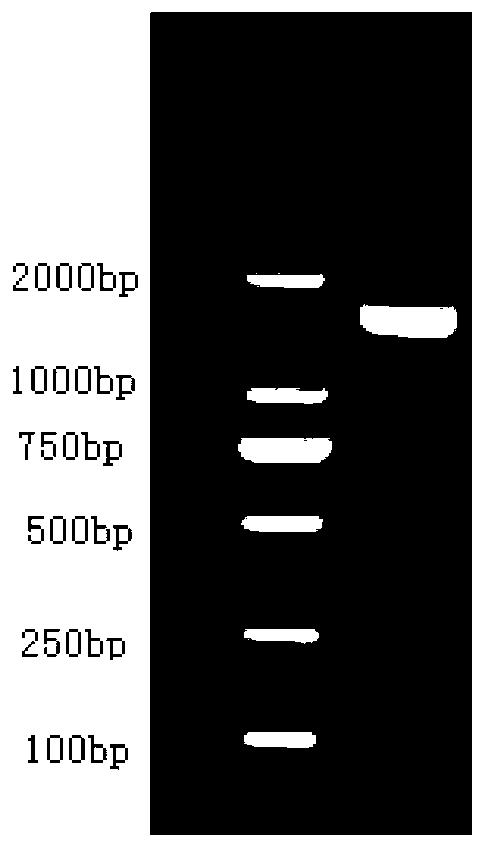
[0037] The TRIzol method was used to extract the total RNA of Lygus japonica, and the total RNA samples with good electrophoretic patterns and OD260 / OD280 values between 1.8 and 2.0 were selected and combined for mRNA purification, and the first-strand cDNA was synthesized by reverse transcription. The PCR reaction system was: 0.5 μg total RNA, 0.5μl Oligo-dT18, 2μl 5× reaction buffer, 2μl 10mM dNTP, 40U RNasin, 200U SuperscriptⅡ, add ddH 2 0 to 10 μl; PCR reaction parameters: 42°C for 30 min, 75°C for 30 min, 4°C for 5 min. Primers ALTre-2-F (5'-GAGTTCTACTACTGGGATTC-3') and ALTre-2-R (5'-GCGTTGGGATAGTCCCCATTG-3') suitable for PCR amplification were designed according to the conserved sequence of known insect membrane-bound trehalase genes , to obtain the conserved sequence of the membrane-bound trehalase gene of Lygus viridans, the conserved sequence is shown in SEQ ID NO: 3 in the sequence listing, the PCR reaction system is...
Embodiment 2
[0039] Embodiment 2: Construction of ALTre-2 gene prokaryotic expression vector
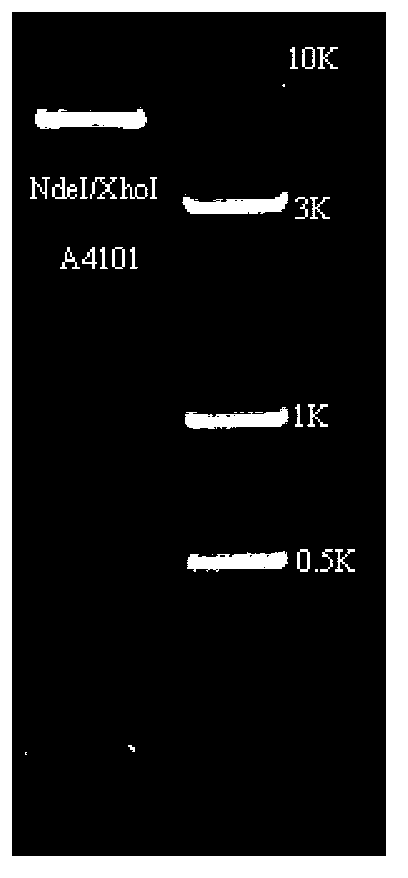
[0040] ALTre-2 gene prokaryotic expression vector construction method: The prokaryotic expression vector construction method containing ALTre-2 gene T vector is as follows: the above sequencing is connected into the pGEM-TEasy vector and pET28a is double-digested with restriction enzymes NdeI and XhoI, Perform large segment joins ( figure 2 ). The enzyme digestion system is: 1.0 μl of each of the two restriction enzymes, 2.0 μl of 10×Buffer buffer, 10.0 μl of gene fragments with appropriate restriction sites, and ddH 2Make up O to 20 μl, and bathe in water at 37°C for 3h. The ligation system is: 5.0 μl of the recovered vector after enzyme digestion, 10.0 μl of the gene fragment, 1.0 μl of T4 DNA ligase, 2.0 μl of 10×Buffer, and ddH 2 Make up O to 20μl, and react at 16°C for 12-16h. After the ligation product was transformed into Escherichia coli TOP10, positive clones were screened by PCR. ...
Embodiment 3
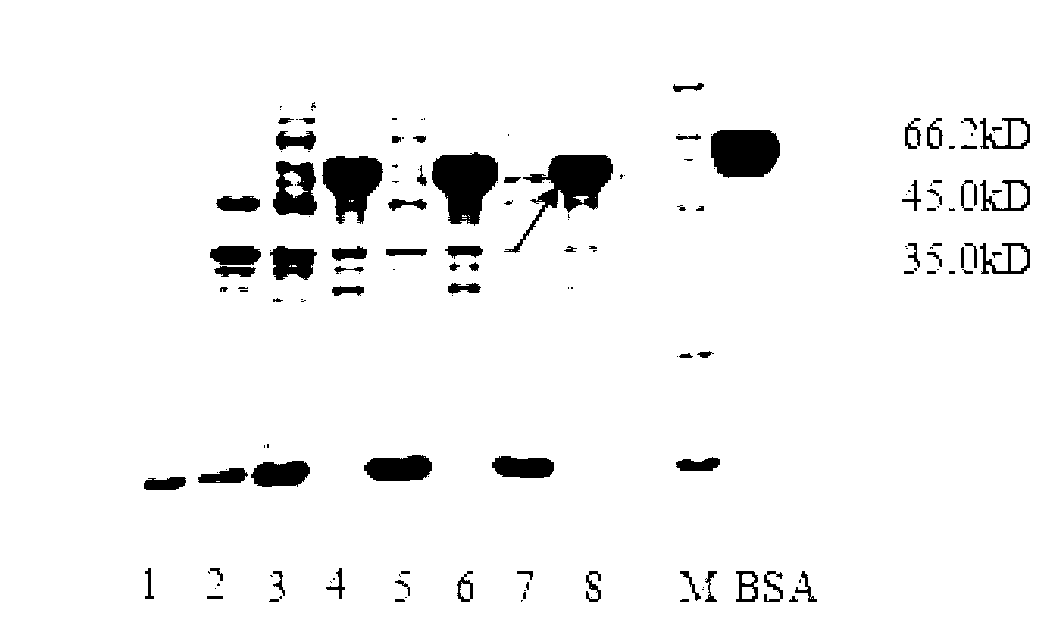
[0042] Example 3: Denaturation and renaturation of ALTre-2 fusion protein and purification of protein
[0043] Denaturation of inclusion body protein was carried out according to the following steps: centrifuge at 4000g, 4°C for 15min, discard the supernatant, wash the bacteria twice with PBS buffer, resuspend in PBS buffer, break the cells under high pressure, centrifuge at 12000g, 4°C for 30min, and save the precipitate For inclusion body, add 60mL binding buffer (6M guanidine hydrochloride, 100mM Tris, 300mM NaCl, pH8.0), resuspend inclusion body, stir to dissolve, centrifuge at 16000g, 4℃ for 30min, keep the supernatant; Elution (buffer A: 6M guanidine hydrochloride, 100mM Tris, 300mM NaCl, pH8.0; buffer B: 8M urea, 100mM Tris, 300mM NaCl, pH8.0; buffer C: 8M urea, 100mM Tris, 300mM NaCl, different concentrations of imidazole, pH8.0); then use 10 times the volume of the column bed Binding buffer (20mM Tris-HCl pH7.9, 10mM imidazole, 0.5M NaCl) to wash the equilibrated colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com