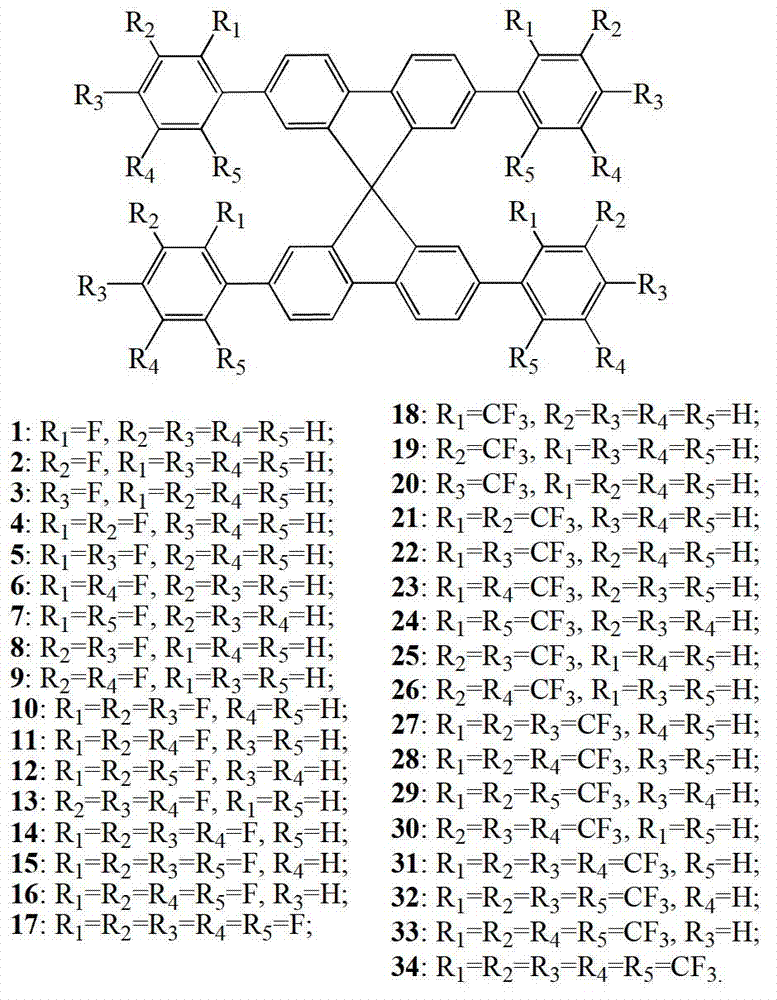
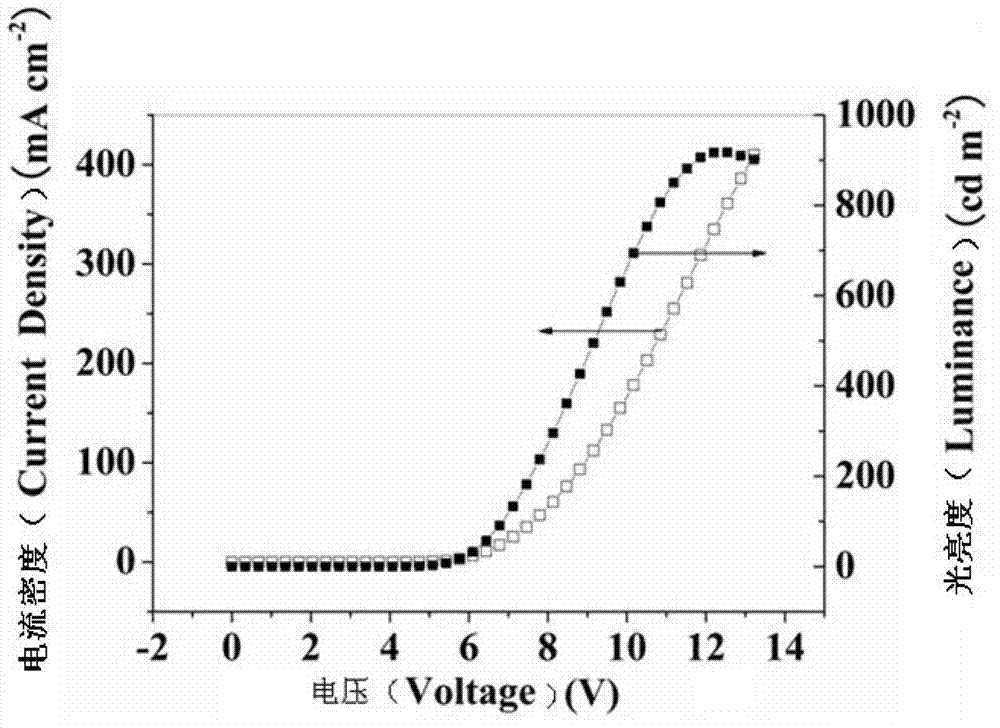
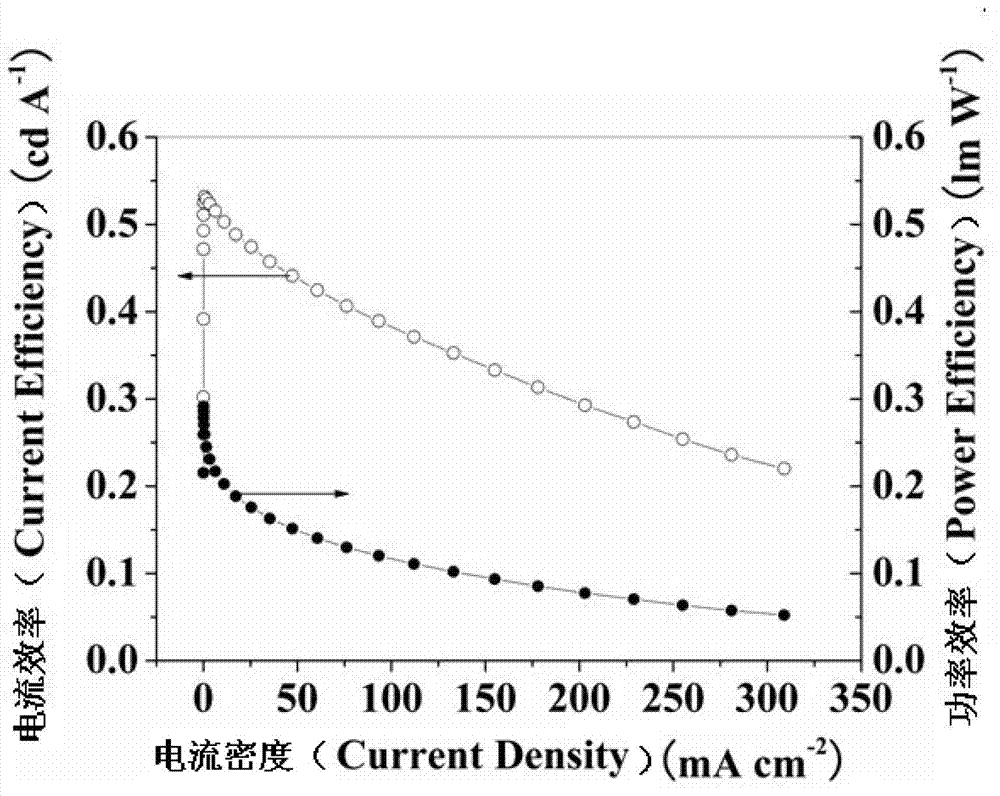
Fluoro-substituted 9, 9'-spirobifluorene blue-light host material, its preparation method and application
A blue-light host material, spirobifluorene technology, which is applied in the direction of luminescent materials, preparation of halogenated hydrocarbons, chemical instruments and methods, etc., can solve the problems of poor performance of blue light devices, difficulties in phosphorescent blue light devices, and difficulty in achieving high efficiency and good color purity Long-life blue light devices and other issues, to achieve good luminous brightness, good thermal stability, and good luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] see figure 1 , Fluorinated 9,9'-spirobifluorene blue-light host material is obtained by ferric chloride-catalyzed bromination of 9,9'-spirobifluorene and bromine at room temperature to obtain reaction intermediates 2,2',7,7' -Tetrabromo-9,9′-spirobifluorene (2,2′,7,7′-tetrabromo-9,9′-spirobifluorene, Spiro-4Br), followed by fluoro- or trifluoromethyl-substituted phenylboronic acid Under the catalysis of tetrakis(triphenylphosphine)palladium, the target product was obtained by Suzuki coupling reaction.
[0034] The intermediate product 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene is synthesized as follows: 9,9′-spirobifluorene (6.52g, 20.6mmol) and anhydrous ferric chloride (FeCl 3 , 10mg) in 60mL of chloroform solution, slowly drop 10mL of chloroform solution containing bromine (4.45mL, 86.6mmol) at 0°C (dropping for more than 1h), spot the plate to follow the reaction (reaction 24h ). After the reaction was completed, the remaining bromine was removed by washing the r...
Embodiment 2
[0036] Example 2: Synthesis of fluorinated 9,9'-spirobifluorene blue light host material 1:
[0037]
[0038] Under nitrogen atmosphere, to 30mL THF and 10mL, 2.0mol·L -1 K 2 CO 3 In the solution, add 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene (1.1g, 1.74mmol), 2-fluorophenylboronic acid (1.40g, 10mmol) and Pd(PPh3) 4 (0.40 g, 0.35 mmol). After the reaction, the mixture was heated to reflux for 24h, and the reaction was tracked by point plate. After the reaction is complete, cool down and add water to stop the reaction. Products with CH 2 Cl 2 Extraction, washing the organic phase with brine, anhydrous MgSO 4 It was dried, concentrated by rotary evaporation to remove the solvent, and 1.05 g of white solid 1 was obtained by column chromatography with ethyl acetate and petroleum ether. 1 H NMR (CDCl 3 ,400MHz):δ6.98(s,4H),7.01-7.10(m,8H),7.18-7.23(m,4H),7.28-7.31(t,J=7.6Hz,4H),7.62(d,J =7.6Hz, 4H), 7.93(d, J=8.0Hz, 4H).
Embodiment 3
[0039] Example 3: Synthesis of fluorinated 9,9'-spirobifluorene blue light host material 5:
[0040]
[0041] Under nitrogen atmosphere, to 30mL THF and 10mL, 2.0mol·L –1 K 2 CO 3 To the solution, 2,2′,7,7′-tetrabromo-9,9′-spirobifluorene (1.1g, 1.74mmol), 2,4di-fluorophenylboronic acid (1.58g, 10mmol) and Pd (PPh 3 ) 4 (0.40g, 0.35mmol). After the reaction, the mixture was heated to reflux for 24h, and the reaction was tracked by point plate. After the reaction is complete, cool down and add water to stop the reaction. Products with CH 2 Cl 2 Extraction, washing the organic phase with brine, anhydrous MgSO 4 It was dried, concentrated by rotary evaporation to remove the solvent, and 1.18 g of white solid 5 was obtained by column chromatography with ethyl acetate and petroleum ether. 1 H NMR (CDCl 3 ,400MHz):δ6.78-6.85(m,8H),7.91(s,4H),7.23-7.25(m,4H),7.57(d,J=8.0Hz,4H),7.93(d,J=8.0 Hz, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com