Detection kit for measuring content of myohemoglobin in serum
A technology for detecting kits and myoglobin, which is applied in the field of medical immunological in vitro diagnosis, can solve the problems of expensive chemiluminescence method and enzyme-linked fluorescence analysis method, long detection cycle of enzyme-linked immunoassay method, and unsuitability for emergency rapid detection, etc. Achieve the effects of high accuracy of measurement results, easy operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
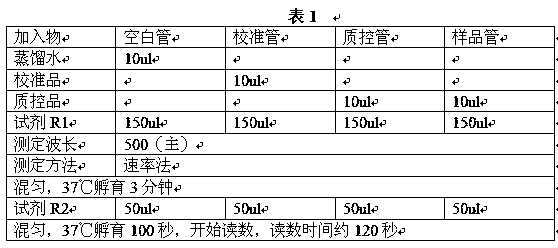
[0031] Embodiment 1: The composition of assay kit is as follows:
[0032] 1. Reagent R1 is:
[0033] PH7.4 phosphate buffer 45mmol / l
[0034] Polyethylene glycol 600080mmol / l
[0035] Disodium EDTA 17mmol / l
[0036] NaN30.9%
[0037] Components can be added sequentially at room temperature, added simultaneously, or individually packaged and prepared just before assay.
[0038] 2. Reagent R2 is:
[0039] Myoglobin polyclonal antibody and polystyrene latex particles are mixed at a mass ratio of 30:100, and the buffer is 0.02M phosphate buffer (pH7.4). After the two are mixed evenly, they are adsorbed at 37°C for 8 hours, and then dialyzed Remove unlinked antibodies, add blocking solution (0.1% skimmed milk powder, 0.2mm glycine), and block for 2 hours. Centrifuge to remove the supernatant, dilute with latex (PH7.4 phosphate buffer 50mmol / l, disodium edetate 16mmol / l, 0.1% skimmed milk powder, 0.9%NaN3) to 0.25%.
[0040] 3. Calibrator preparation:
[0041] Recombinant ...
Embodiment 2
[0044] Embodiment 2: Correlation test of detection reagent
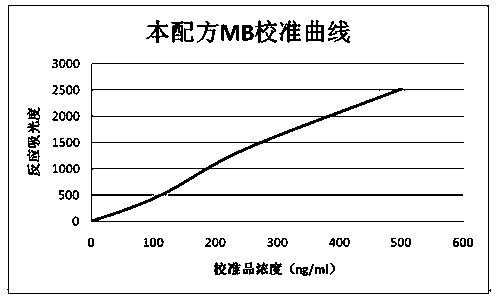
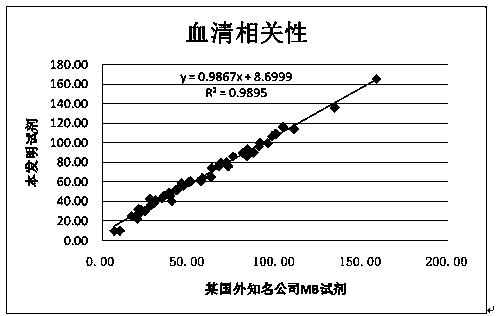
[0045] Use the inventive reagent of this method (the specific formula is the same as that in Example 1) and the MB latex enhanced reagent of the contrast reagent A company, and use the automatic 7170 automatic biochemical analyzer to conduct simultaneous tests on 50 human sera (including normal and abnormal samples) according to their respective parameters. Determination and correlation analysis of the measured values. Determination is carried out according to the parameters in the above "MB determination method", and the determination results are shown in figure 2 , X and Y axes are measured values (MB content ng / ml),
[0046] Depend on figure 2 The results show that the correlation between the two reagents is R 2 =0.9895, the regression equation is y=0.9867x+8.6999. The results show that this reagent has a good correlation with imported reagents in the determination of patient serum, and has good specificit...
Embodiment 3
[0048] Embodiment 3: minimum detection limit test
[0049] The purpose of this experiment is to detect the minimum detection sensitivity of the reagent when testing clinical samples.
[0050] Use experimental example 1 reagent, control reagent, standard, blank solution (normal saline and purified water), normal human serum samples, and low-value samples (samples whose values are within ±1 / 3 of the lower limit of the linear range of the reagent).
[0051] Machine: Hitachi 7170 automatic biochemical analyzer.
[0052] Operation steps: Use physiological saline or deionized water to dissolve low-value samples, then dilute 50% to 5 points, test each sample 5 times with the zero point, calculate the average value, and obtain the SD value.
[0053] Result analysis: According to the test data, calculate the SD value and CV value, respectively calculate 1SD, 2SD, starting from the smallest, the value of the average - 2SD is above the zero point average value + 2SD is the minimum det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Ph | aaaaa | aaaaa |
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com