Intermediate compound used for preparing Ambrisentan, preparation method thereof, and preparation of Ambrisentan
A compound and an independent technology, applied in the field of preparation of ambrisentan and intermediate compounds of ambrisentan, can solve the problems of low resolution yield, increased cost, low atom utilization rate and the like, and achieve high yield, The effect of simple operation, improved atom utilization and synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
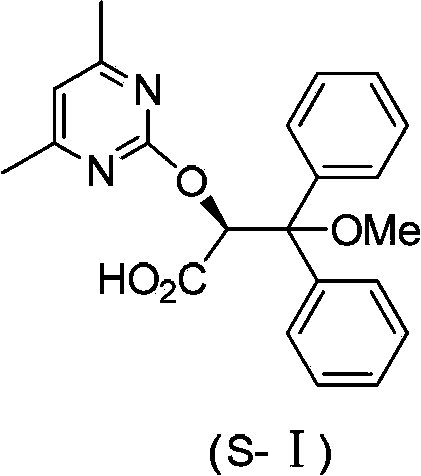
[0079] The present invention provides an intermediate compound IV for the preparation of ambrisentan and its enantiomers, the structural formula of which is as follows:
[0080]
[0081] Ambrisentan is prepared by adopting the intermediate without splitting, and the atom economy is high.
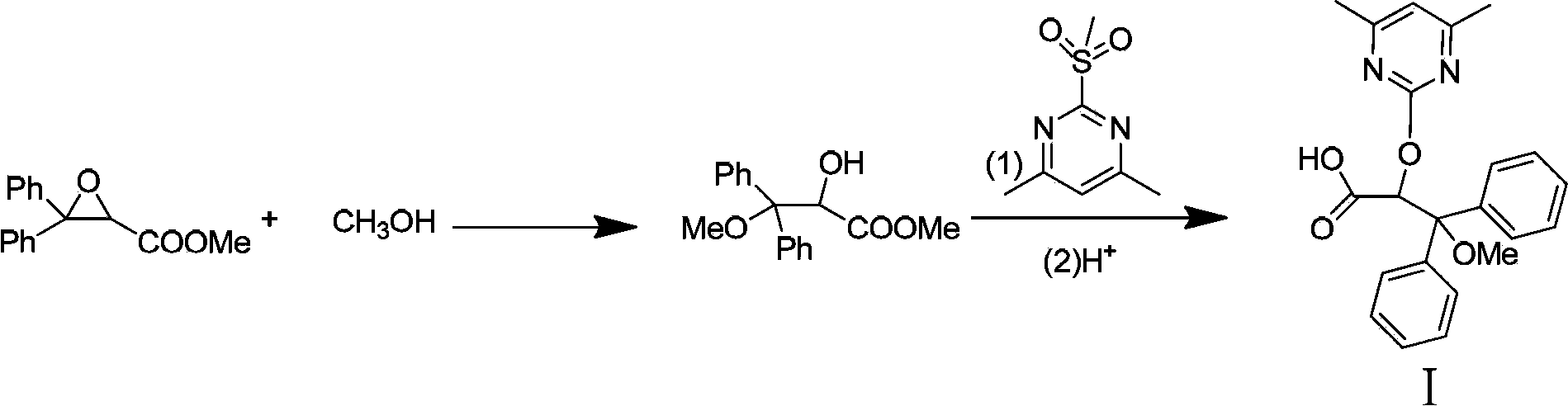
[0082] The present invention further provides a method for preparing the compound shown in formula IV, the method comprising removing the protecting group from the compound shown in formula III in the presence of a reaction solvent to obtain the compound shown in formula IV,
[0083]
[0084] Among them, R 1 , R 2 each independently selected from a hydrogen atom, C 1 -C 6 Alkyl, C 1 -C 6 Haloalkyl, phenyl or C 1 -C 6 Alkoxy, halogen, hydroxy-substituted phenyl, or R 1 and R 2 Together with the carbon atom they are attached to form C 3 -C 6 Cycloalkyl, more preferably, R 1 and R 2each independently selected from a hydrogen atom, methyl, ethyl, propyl, tert-butyl, trichlorom...
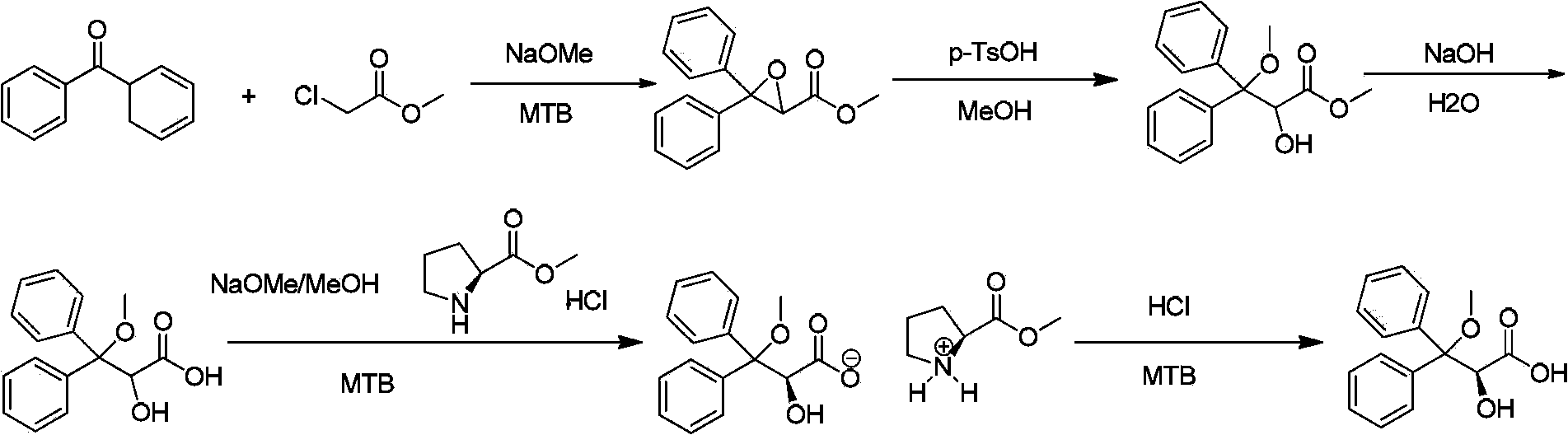
Embodiment 1a
[0128] Weigh 80g (0.44mol) of mannitol and 480g (3.5mol) of zinc chloride, dissolve in 1000ml of acetone, and stir at 20°C for 14h. TLC (PE: EA = 1: 2) monitored that the reaction was complete. Weigh 500g of potassium carbonate and dissolve it in 1000ml of water, add the aqueous solution of potassium carbonate and 1.5L of dichloromethane into the system and stir for 30min, filter and wash the filter residue with 100mL of dichloromethane, extract the aqueous phase with 500ml of dichloromethane, and combine the organic phases , the organic phase was washed once with saturated brine 300mL, anhydrous Na 2 SO 4 dry. After filtration, the filtrate was spin-dried to obtain 125.2 g of a pale yellow solid (Compound X-a). Add 150ml of isopropyl ether and 100ml of petroleum ether, heat at 80°C, and cool to room temperature to obtain 49g of white solid. Yield 43%. Its structural identification data are as follows:
[0129] MS: [M+H] + : 263.18[M+Na] + : 303.22.
Embodiment 2a
[0131] Weigh 48.3g (0.18mol) of (Compound X-a) and dissolve it in 480mL of dichloromethane. Add water 33ml and NaIO in turn 4 49.5g (0.23mol), stirred and reacted at 20°C for 5h. TLC (PE: EA = 1: 1) monitored that the reaction was complete. After filtration and rotary evaporation at 40°C, add (methanol:water=9:1) mixed solution 500ml and sodium bicarbonate 70g (0.83mol), add bromine 18.8ml (0.36mol) dropwise in ice-water bath, and stir at 20°C Overnight, TLC (PE: EA = 4: 1) detection, phosphomolybdic acid color development, add saturated sodium thiosulfate 15ml in ice water bath, the system completely faded, add 700ml of dichloromethane, 300ml of water to separate, the organic phase with saturated Wash once with 300 mL of saline, anhydrous Na 2 SO 4 dry. After filtering, the filtrate was spin-dried to obtain 44.3 g of a colorless liquid (Compound XI-a).
[0132] MS:[M+H] + :161.08;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com