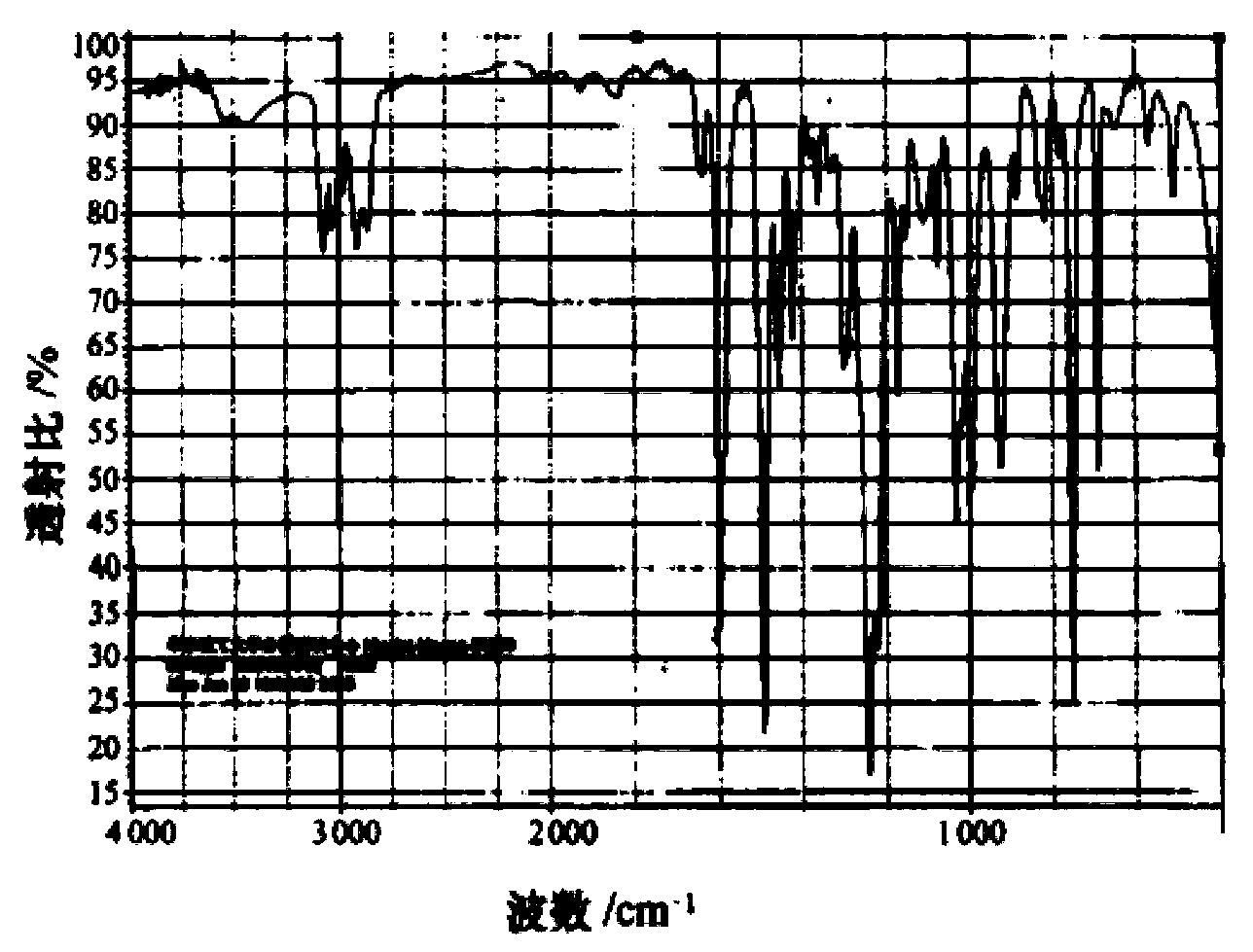
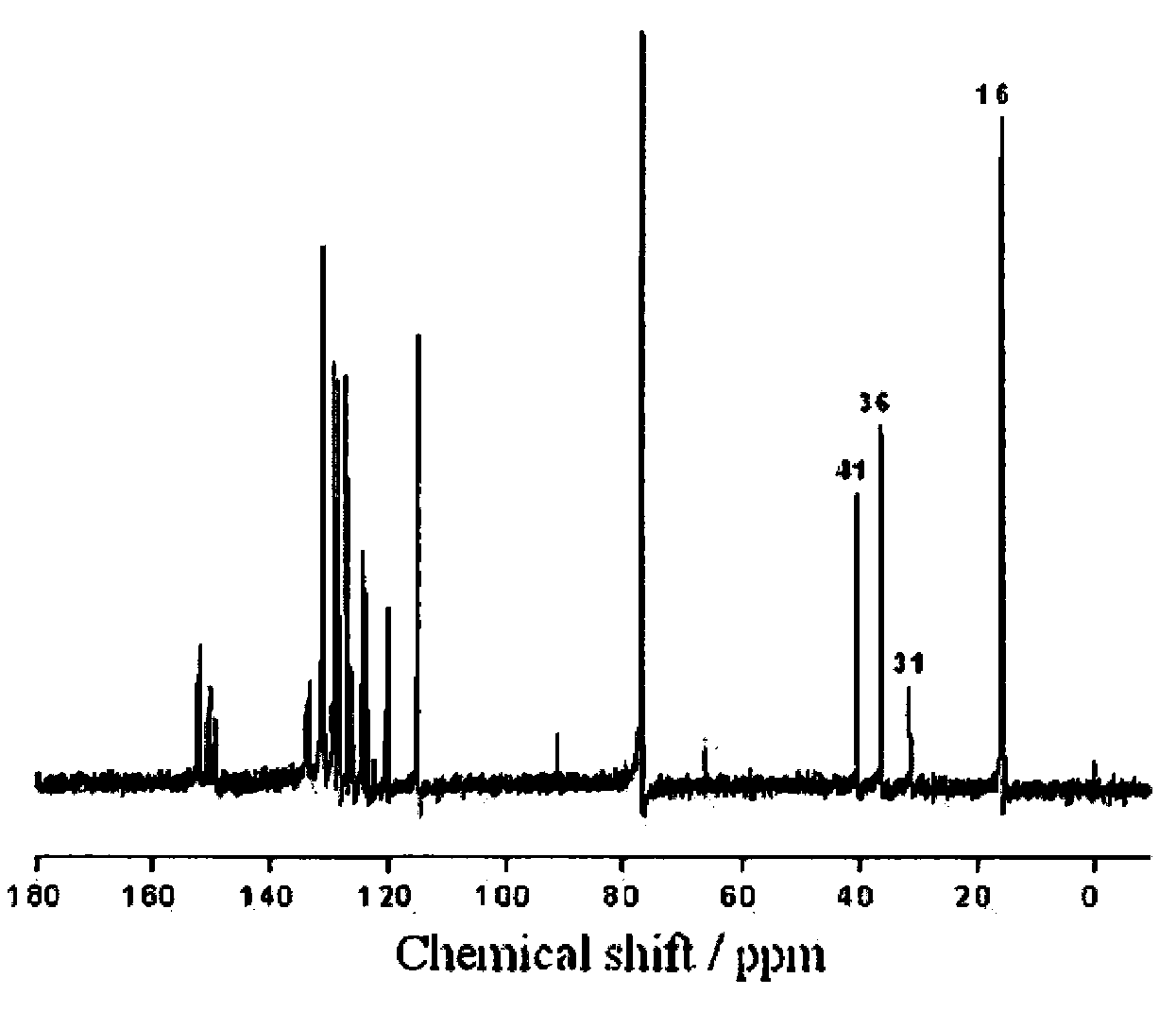
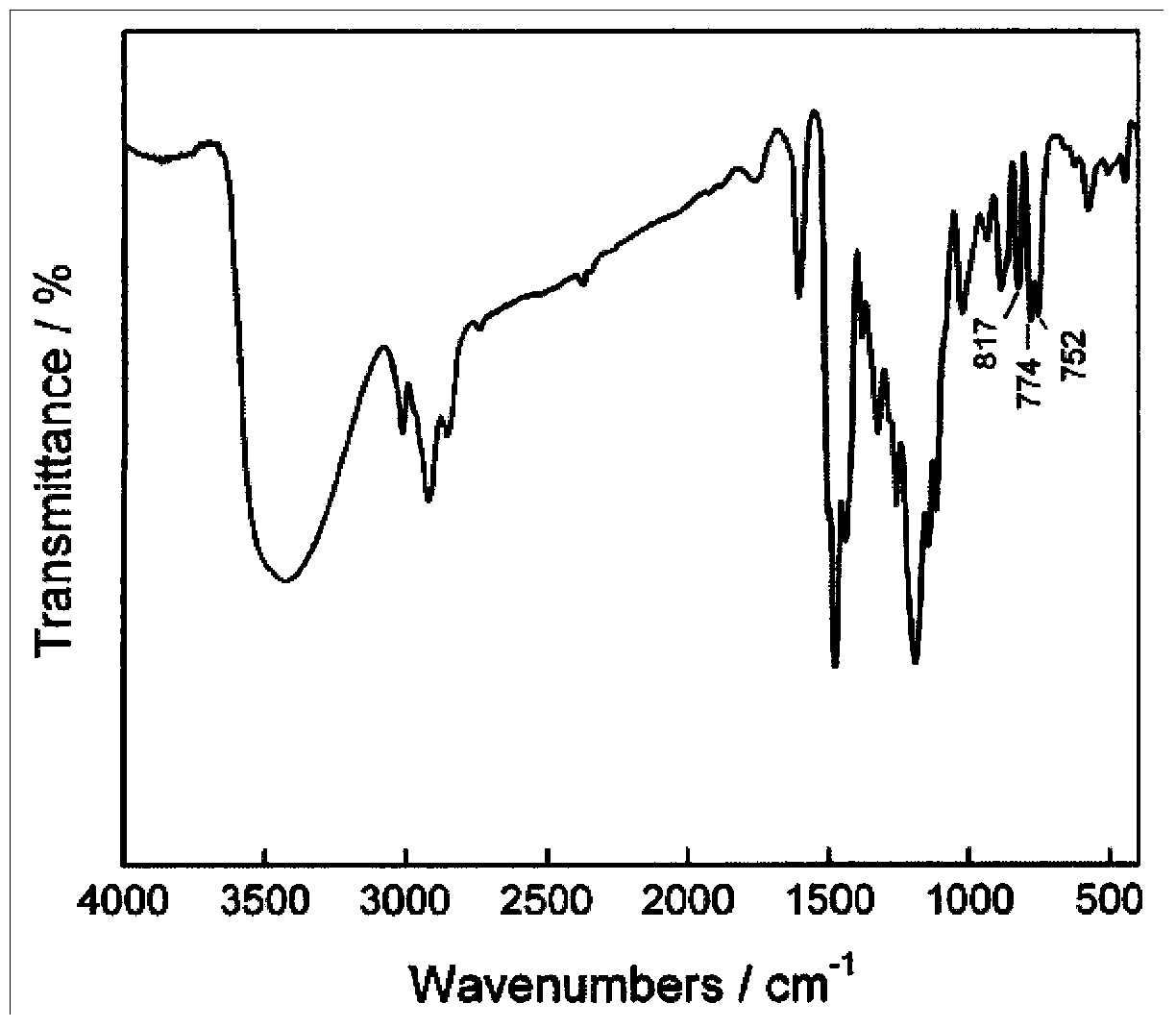
Synthesis method of phenolic epoxy resin
A novolac epoxy resin and a synthesis method technology, which are applied in the field of organic synthesis and can solve the problems that the closed-ring epoxidation reaction cannot be fully carried out, cannot meet the technical requirements of high-purity novolac epoxy resin, and is easy to emulsify.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1: the synthesis of novolac epoxy resin
[0152] The implementation steps of this embodiment are as follows:
[0153] Step A: Preparation of Alkenyl Phenyl Ether
[0154] Under the conditions of normal pressure and temperature of 10°C, in an inert atmosphere of nitrogen, let the phenolic compound of formula (I), R in its formula (I) 1 , R 3 is hydrogen, R 2 It is methyl, and sodium hydroxide catalyst A is dissolved in acetone solvent A to obtain a solution containing phenol. The amount of sodium hydroxide catalyst A is 0.1% of the phenolic formula (I) phenol compound weight. The amount of acetone solvent A is 3 times of the total weight of phenol and sodium hydroxide. Then, add 3-chloropropene (II) haloalkene compound into the solution, and the molar ratio of phenol to 3-chloropropene is 1:4.5.
[0155] Next, in the presence of sodium hydroxide catalyst A at a temperature of 65°C, let 3-chloropropene react with phenol for 0.5 h, then add chloroform extr...
Embodiment 2
[0163] Embodiment 2: the synthesis of o-butyl novolac epoxy resin
[0164] The implementation steps of this embodiment are as follows:
[0165] Under the conditions of normal pressure and temperature of 82°C, in a nitrogen inert atmosphere, let 2-butylphenol formula (I) phenol compound, the R in its formula (I) 1 , R 3 is hydrogen, R 2 Be butyl, be dissolved in 2-butanone solvent A with potassium hydroxide catalyst A, obtain a kind of solution that contains 2-butylphenol, the amount of potassium hydroxide catalyst A is 2-butylphenol formula (I) phenol 10.0% by weight of compound. The amount of 2-butanone solvent A is 3.2 times of the total weight of 2-butylphenol and potassium hydroxide. Then, add 3-bromopropene formula (II) haloalkene compound into the solution, and the molar ratio of 2-butylphenol to 3-bromopropene is 1:5.6.
[0166] Next, in the presence of potassium hydroxide catalyst A at a temperature of 70°C, let 3-bromopropene react with 2-butylphenol for 10.0 h, ...
Embodiment 3
[0174] Embodiment 3: the synthesis of o-isopropyl novolac epoxy resin
[0175] The implementation steps of this embodiment are as follows:
[0176] Step A: Preparation of Alkenyl Phenyl Ether
[0177] Under the conditions of normal pressure and temperature of 65°C, in an argon inert atmosphere, let 6-isopropylphenol formula (I) phenol compound, formula (I) in R 1 is isopropyl, R 2 , R 3 It is hydrogen, and calcium hydroxide catalyst A is dissolved in 2-pentanone solvent A to obtain a solution containing 6-propophenol. The amount of calcium hydroxide catalyst A is 0.8% of 6-propophenol weight. The amount of 2-pentanone solvent A is 4.0 times of the total weight of 6-isopropylphenol and calcium hydroxide. Then, add 4-bromobutene formula (II) haloalkene compound into the solution, and the molar ratio of 6-isopropylphenol to 4-bromobutene is 1:0.5.
[0178] Next, in the presence of a calcium hydroxide catalyst A at a temperature of 24°C, let 4-bromobutene react with 6-isopro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com