Method for removing metal ion purity of rare earth aqueous solution extraction
A technology of impurity metal ions and rare earth ions, applied in the direction of improving process efficiency, etc., can solve the problem of uncontrollable three-phase distribution of non-rare earth impurity ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
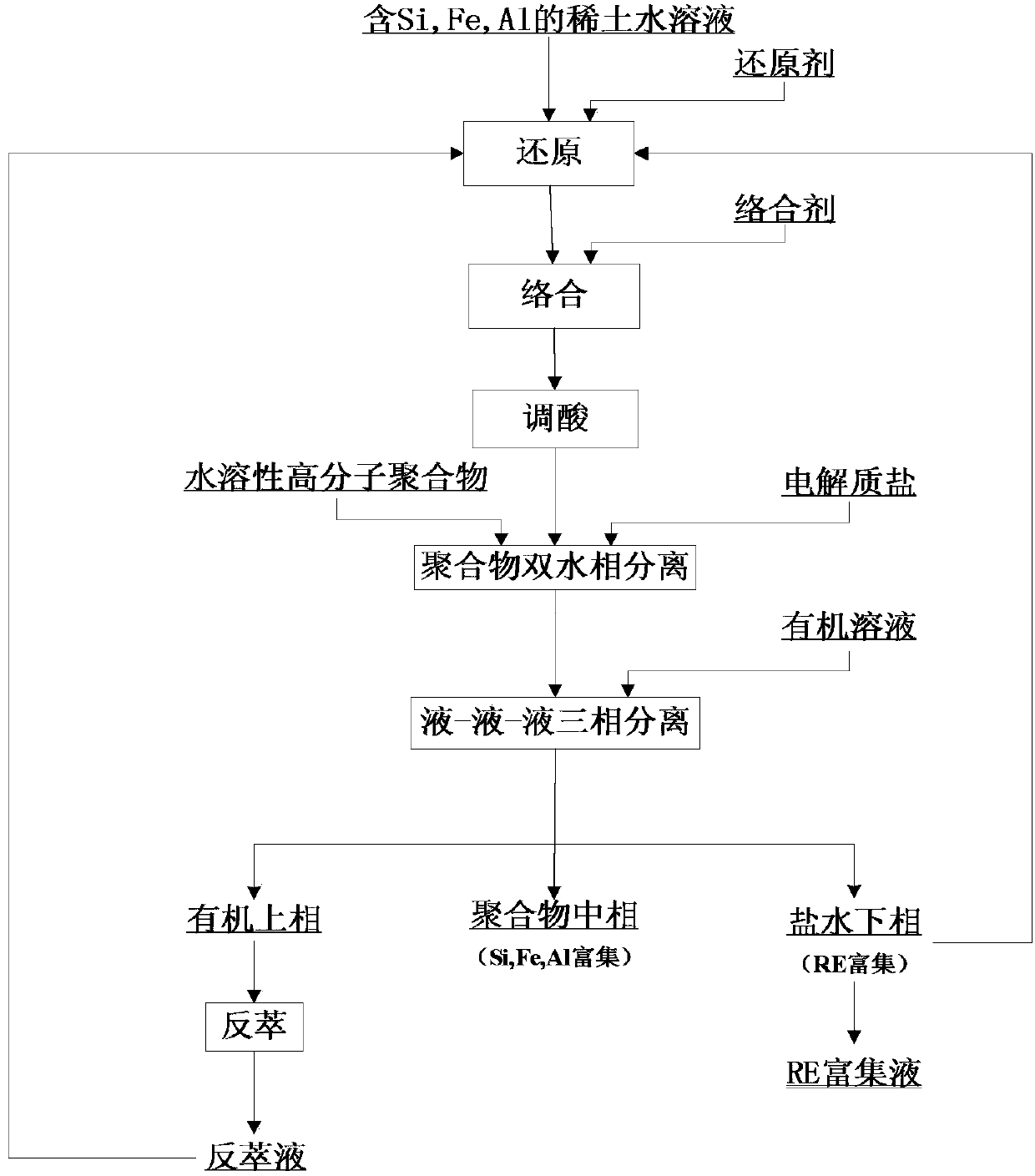
[0041]Dissolve the oxides of lanthanum, samarium, and thulium with 7mol / L aqueous hydrochloric acid solution, heat the acid, and prepare a mother solution containing lanthanum, samarium, and thulium ions (concentration is about 5mmol / L). Dissolve aluminum chloride hexahydrate to prepare mother liquor containing aluminum ions (concentration is about 30mmol / L); dissolve ferric chloride to prepare iron-containing mother liquor (concentration is about 15mmol / L); dissolve sodium silicate solid to prepare Silicon-containing mother liquor (concentration is about 25mmol / L). Take 1 mL of each of the above-mentioned mother liquors, add hydroxylamine hydrochloride in a molar ratio of 0.3:1 to the total amount of impurity metal ions aluminum, iron and silicon as a reducing agent, and mix thoroughly at room temperature. 1,10-phenanthroline was dissolved to make a stock solution with a concentration of 35mmol / L, and EDTA solid was dissolved with 1mol / L NaOH solution to make an aqueous solut...
Embodiment 2
[0043] Dissolve the oxides of cerium, samarium and lutetium with 6mol / L nitric acid aqueous solution respectively, heat and chase the acid, and prepare mother liquor containing cerium, samarium and lutetium ions (concentration is about 5mmol / L). Dissolve aluminum chloride hexahydrate to prepare mother liquor containing aluminum ions (concentration is about 30mmol / L); dissolve ferric chloride to prepare iron-containing mother liquor (concentration is about 15mmol / L); dissolve sodium silicate solid to prepare Silicon-containing mother liquor (concentration is about 25mmol / L). Take 1 mL of each of the above mother liquors, add hydroxylamine hydrochloride in a molar ratio of 0.7:1 to the total amount of impurity metal ions aluminum, iron and silicon as a reducing agent, and mix thoroughly at room temperature. 1,10-phenanthroline was dissolved to make a stock solution with a concentration of 35mmol / L, and EDTA solid was dissolved with 1mol / L NaOH solution to make an aqueous solutio...
Embodiment 3
[0045] Dissolve the oxides of lanthanum, cerium, gadolinium, and thulium with 6mol / L perchloric acid aqueous solution respectively, heat the acid, and prepare a solution containing lanthanum, cerium, gadolinium, and thulium ions (concentration is about 5mmol / L). Dissolve aluminum chloride hexahydrate to prepare mother liquor containing aluminum ions (concentration is about 30mmol / L); dissolve ferric chloride to prepare iron-containing mother liquor (concentration is about 15mmol / L); dissolve sodium silicate solid to prepare Silicon-containing mother liquor (concentration is about 25mmol / L). Take 1 mL of each of the above mother liquors, add hydroxylamine hydrochloride in a molar ratio of 0.5:1 to the total amount of impurity metal ions aluminum, iron and silicon as a reducing agent, and mix thoroughly at room temperature. Dissolve 1,10-phenanthroline to make an aqueous solution with a concentration of 35mmol / L, and dissolve EDTA solid with 1mol / L NaOH solution to make an aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com