Alpha-MSH analogue with therapeutic activity
A -NH2, CH3-C technology, applied in the field of peptide analogs of α-melanocyte-stimulating hormone (α-MSH), which can solve the problem of low protease sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment approach 1
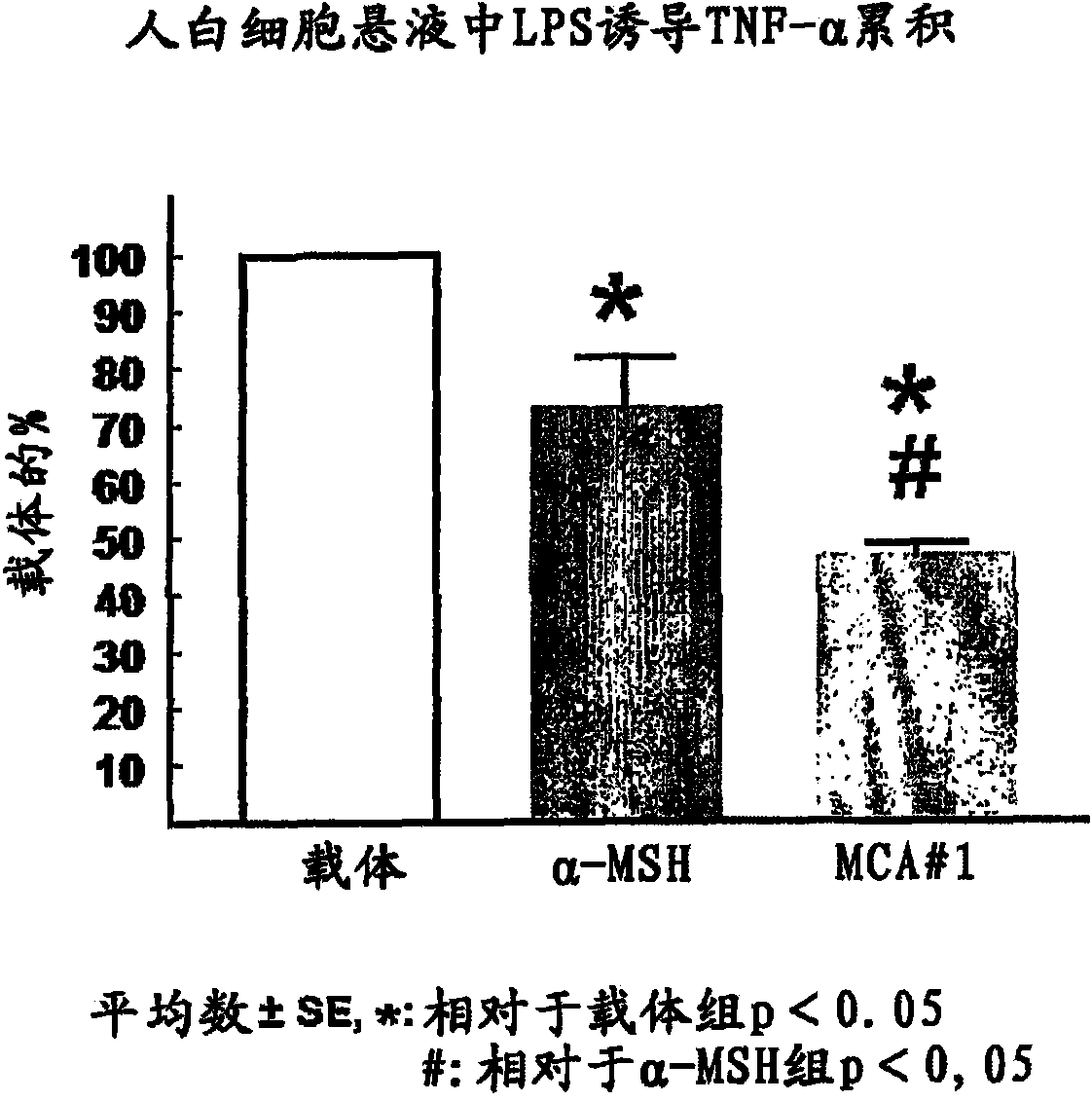
[0327] Inhibition of TNF-α production induced by LPS produced by human leukocytes in vitro
[0328] 20 mL of human blood was collected in a vacuum blood collection tube (vacutainer tube) containing EDTA. Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Paque Plus from Amersham's Instruction 71-7167-00AD, 2002-06. PBMCs were counted using trypan blue solution (Sigma) and counted at 5 x 10 5 Cells / mL concentration PBMC were incubated in RPMI1640 (Applichem) supplemented with 10 mM hydroxyethylpiperazineethanesulfonic acid (Hepes) (Sigma), 2 mM L-glutamine (Sigma ), 0.1% bovine serum albumin (BSA) (Sigma), and 50 U / 50 μg / mL penicillin / streptomycin (Sigma). at 5% CO 2 Isolated PBMCs were incubated in 24-well flat bottom plates (Corning Incorporated) with culture medium, 10 ng LPS / mL (Sigma) and test compounds at 37°C in an atmosphere of , 95% air. After 18 hours, samples were centrifuged and assayed using Tumor Necrosis Factor Alpha [(h)TNF-α] from the Huma...
experiment approach 2
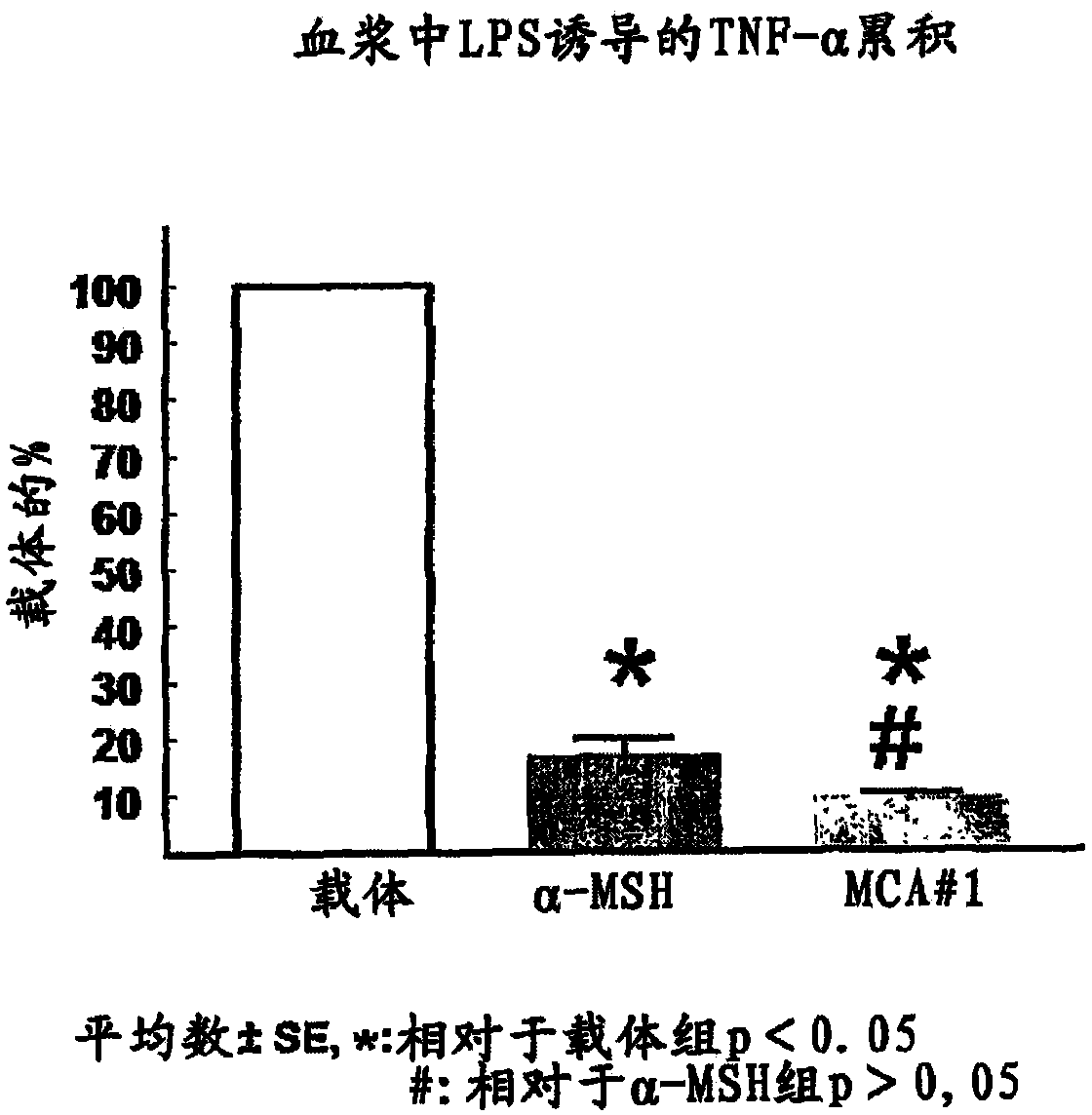
[0342] Inhibition of LPS-induced TNF-α production in rats
[0343] Experimental animals: Female Wistar rats (220-240g) were from Charles River (Charles River), Sulzfeld, Germany, and made to live in a temperature (22-24°C) and humidity (40-70%) controlled environment with a 12-hour Inside a room with a light-dark cycle (lighting from 6:00A.M. to 6:00P.M.). Rats were maintained on a standard rodent chow (with 140mmol / kg sodium, 275mmol / kg potassium and 23% protein, Altromin International, Lage, Germany) with free access to a water source.
[0344] Animal preparation: Under isoflurane-nitrous oxide anesthesia, durable medical grade polyethylene (Tygon) catheters were implanted into the abdominal aorta and inferior vena cava via the femoral artery and vein, respectively. Following implantation, animals were housed individually for 7-10 days until the day of the experiment.
[0345] Experimental procedure: Prior to the experiment, all rats were acclimated to the confinement cage...
experiment approach 3
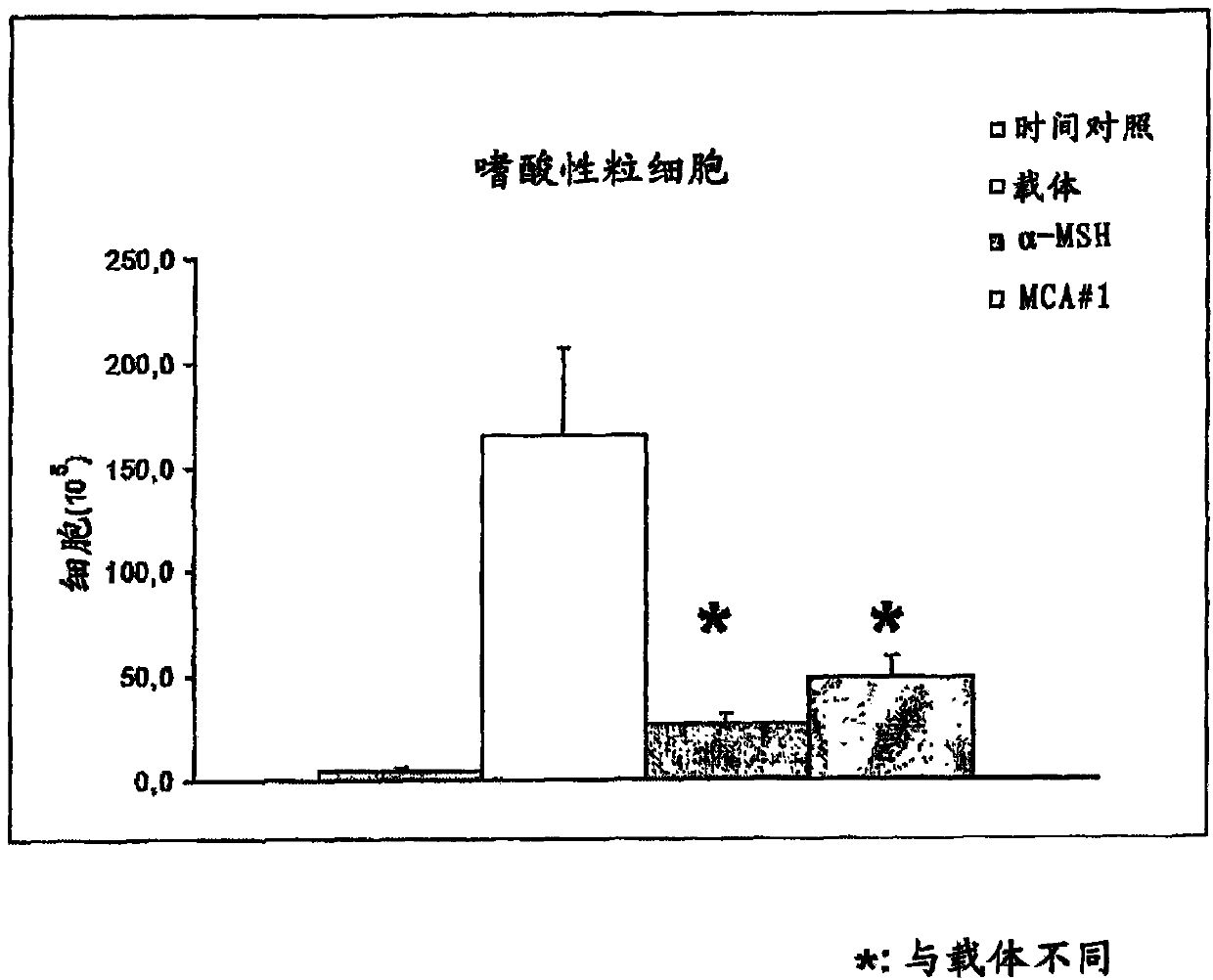
[0354] Inhibition of neutrophil and eosinophil infiltration after LPS inhalation in rats
[0355] Male Sprague-Dawley rats from M&B A / S, DK-8680Ry, Denmark were used for all experiments. Rats were housed in type 3 standard cages in a temperature (22-24°C) and humidity (40-70%) controlled environment with a 12-hour light-dark cycle (from 6:00 A.M. to 6:00 A.M. :00P.M. for lighting). The feed was a special formula of autoclaved Altromin 1324 produced by Altromin Denmark (Chr. Pedersen A / S, 4100 Ringsted, Denmark). Feed and water were given ad libitum.
[0356] Rats were randomly assigned to experimental groups after acclimatization and test compounds were dosed intravenously at the beginning of LPS induction and again 8 hours after induction of LPS.
[0357]Rats in group 3 were anesthetized with 0.1 ml fentanyl / domacar / 100 g and dosed with the test compound intravenously. Immediately after dosing they were placed into a breathing chamber where they were sprayed with LPS solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com