A borate-doped titanium lithium phosphate two-component surface-modified iron fluoride positive electrode material and preparation method thereof
A surface modification, lithium titanium phosphate technology, applied in battery electrodes, electrochemical generators, electrical components, etc., can solve the problems of high reaction activation energy, high conversion reaction, poor economy, etc., to overcome the ionic conductivity Very low, improved electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
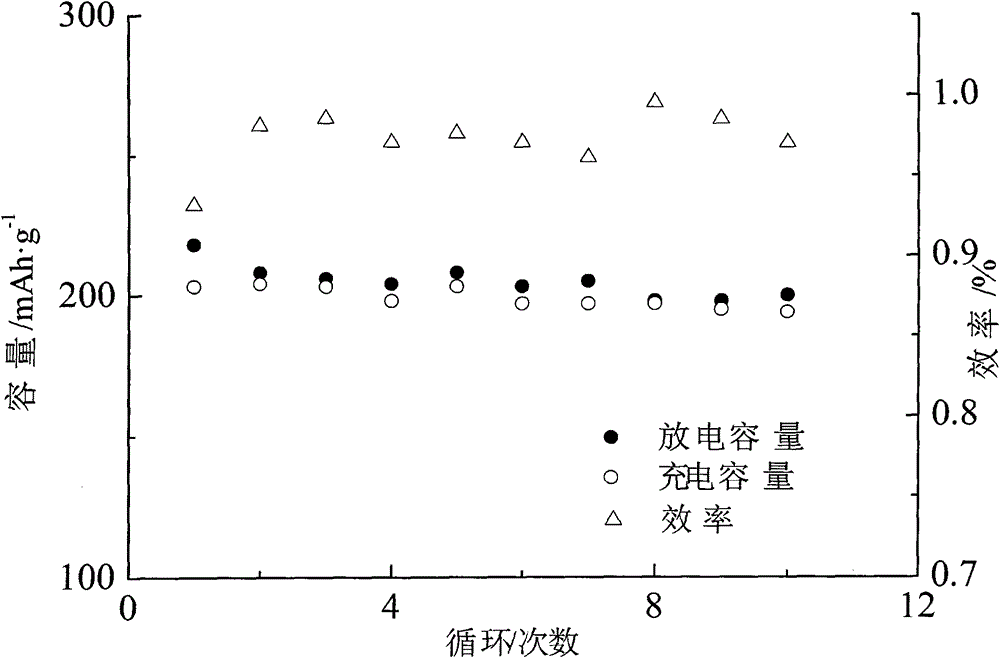
[0019] Embodiment 1: Al 2 o 3 : SiO 2 : TiO 2 : NH 4 h 2 PO 4 : Li 2 CO 3 Mix evenly in the ratio of 0.05:0.2:1.9:2.8:0.65 (molar ratio), add 3.5% of 95% ethanol, and ball mill for 12 hours at a speed of 110 rpm in a ball mill. Dry in a vacuum oven at 15 Pa for 2.5 hours, take it out and re-grind it in an agate mortar for 15 minutes, and heat up the ground powder to 650°C at a rate of 6°C / min for 6 hours to make Li 1.3 Al 0.1 Ti 1.9 8i 0.2 P 2.8 o 12 Solid electrolyte powder. Fe(NO 3 ) 3 9H 2 O and ammonium fluoride (1.0:3.1 molar ratio) with 3.2% by weight Li 1.3 Al 0.1 Ti 1.9 Si 0.2 P 2.8 o 12 The solid electrolyte powder, Tween-80 with 0.6% by weight and diethanolamine borate with 0.6% by weight are ball milled at room temperature for 5 hours under the protection of high-purity nitrogen in a high-energy ball mill. Under the protection of the mixed gas of % argon, the temperature is raised to 300 degrees and the temperature is kept at 2 hours, and the...
Embodiment 2
[0020] Embodiment 2: Al 2 o 3 : SiO 2 : TiO 2 : NH 4 h 2 PO 4 : Li 2 CO 3 Mix evenly in the ratio of 0.05:0.2:1.9:2.8:0.65 (molar ratio), add 8% 95% ethanol, and ball mill for 45 hours at a speed of 450 rpm in a ball mill. Dry in an 80Pa vacuum oven for 8 hours, take it out and re-grind in an agate mortar for 25 minutes, and the ground powder is heated to 900℃ at a rate of 25℃ / min and kept for 15 hours to make Li 1.3 Al 0.1 Ti 1.9 Si 0.2 P 2.8 o 12 Solid electrolyte powder. FeCl 3 ·6H 2 O and ammonium fluoride (1.0:3.6 molar ratio) with 13% by weight Li 1.3 Al 0.1 Ti 1.9 Si 0.2 P 2.8 o 12 The solid electrolyte powder, 2.8% by weight of span-60 and 3.0% by weight of aminotriethyl borate were ball milled at room temperature for 20 hours under the protection of high-purity argon in a high-energy ball mill, and then the materials were taken out and mixed with 5% hydrogen and Under the protection of a mixed gas of 95% argon, the temperature was raised to 450 ...
Embodiment 3
[0021] Embodiment 3: Al 2 o 3 : SiO 2 : TiO 2 : NH 4 h 2 PO 4 : Li 2 CO 3 Mix evenly at a ratio of 0.05:0.2:1.9:2.8:0.65 (molar ratio), add 5% of 95% ethanol, and ball mill in a ball mill at a speed of 200 rpm for 25 hours. Dry in a vacuum oven at 60 Pa for 7 hours, take it out and re-grind in an agate mortar for 20 minutes, and the ground powder is heated to 750°C at a rate of 20°C / min and kept for 12 hours to make Li 1.3 Al 0.1 Ti 1.9 Si 0.2 P 2.8 o 12 Solid electrolyte powder. Will Fe 2 (SO 4 ) 3 9H 2 O and ammonium fluoride (molar ratio 1.0:3.5) with 7% by weight Li 1.3 Al 0.1 Ti 1.9 Si 0.2 P 2.8 o 12 The solid electrolyte powder, tx-10 with a weight percentage of 2.0% and 2-methylamino-5-pyridine borate with a weight percentage of 2.1% were ball milled at room temperature for 15 hours under the protection of high-purity nitrogen in a high-energy ball mill, and then the materials were taken out. Under the protection of a mixed gas of 5% hydrogen and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com