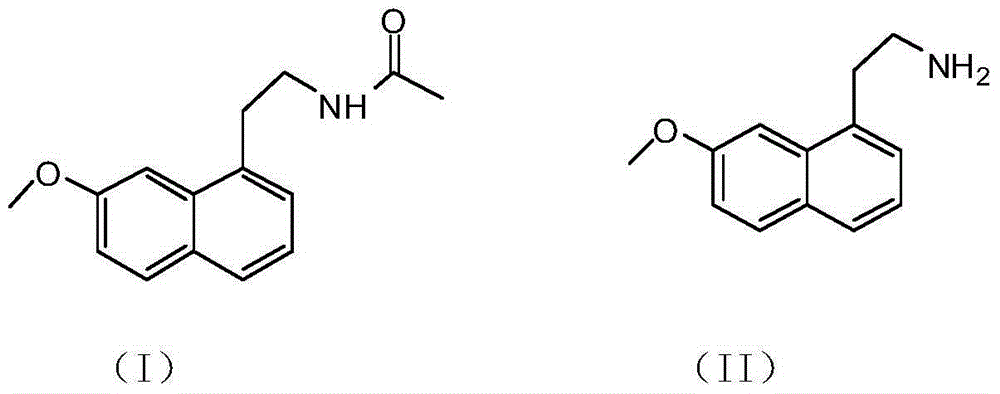
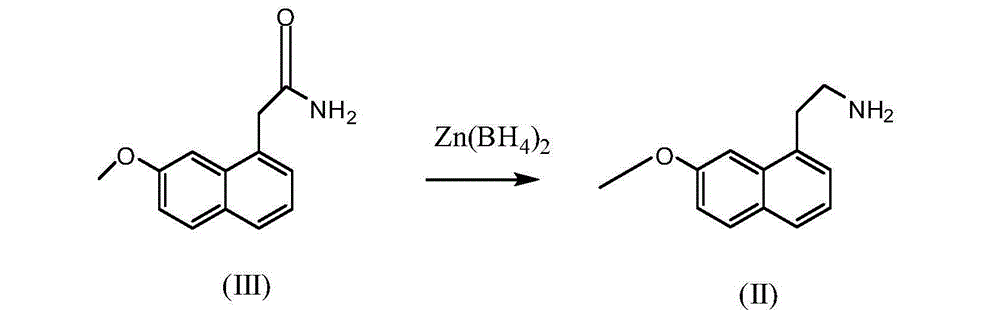The preparation method of 2-(7-methoxyl-1-naphthyl)ethylamine
A methoxyl and naphthyl technology, which is applied in the field of preparation of agomelatine key intermediate 2-(7-methoxy-1-naphthyl)ethylamine, can solve the problem of potential safety hazards and the overall yield Low cost, high production cost and other issues, to achieve the effect of no safety hazard, short reaction route and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In the reaction flask, add 360ml of tetrahydrofuran, ZnCl 2 45.60g (0.335mol) and KBH 4 36.2g (0.67mol), stirring at room temperature for 2 hours; adding 36g (0.167mol) of 2-(7-methoxy-1-naphthyl)acetamide and 360ml of toluene, heating slowly, steaming out THF in the system, making the internal Temperature reaches 110°C, heat preservation and stirring for 8 hours;
[0039] Cool to room temperature, slowly drop ice water into the reaction system to terminate the reaction, pour the reaction solution into 200ml of 10% hydrochloric acid aqueous solution by weight, the pH value of the reaction system is about 2, filter, the filtrate is separated, and the toluene layer is discarded. The aqueous layer was basified with a 20% weight concentration sodium hydroxide solution to a pH of 11-12. Extract twice with 200ml of ethyl acetate, combine the extracts with MgSO 4 After drying, filtering, and evaporating to dryness, 32.0 g of light yellow oily substance 2-(7-methoxy-1-naphth...
Embodiment 2
[0041]In the reaction flask, add 2-methyltetrahydrofuran 360ml, ZnCl 2 34.08g (0.25mol) and KBH 4 27.0g (0.50mol), stirred at room temperature for 4 hours. 36 g (0.167 mol) of 2-(7-methoxy-1-naphthyl) acetamide and 360 ml of toluene were heated slowly to evaporate THF in the system to bring the internal temperature to 70°C and keep stirring for 6 hours. Cool to room temperature, slowly drop ice water into the reaction system to terminate the reaction, pour the reaction solution into 200ml of sulfuric acid aqueous solution with a weight concentration of 10%, the pH value of the reaction solution is between about 1, filter, the filtrate is separated, and the toluene layer discard. The aqueous layer was alkalized to pH 10 with 10% potassium hydroxide solution by weight. Extract with dichloromethane 150ml×2, combine the extracts with NaSO 4 Dry, filter, and evaporate to dryness to obtain 25.5 g of light yellow oily substance 2-(7-methoxy-1-naphthyl)ethylamine, yield 75.89%, MS...
Embodiment 3
[0043] In the reaction flask, add 360ml of tetrahydrofuran, ZnCl 2 22.76g (0.167mol) and KBH 4 18.04g (0.334mol), stirring at room temperature for 2 hours; adding 36g (0.167mol) of 2-(7-methoxy-1-naphthyl) acetamide and 360ml of toluene, heating slowly, distilling THF in the system, making the internal temperature reached 100°C, and kept stirring for 3 hours. Cool to room temperature, slowly drop ice water into the reaction system to terminate the reaction, pour the reaction solution into 300ml of 10% hydrochloric acid aqueous solution by weight, the pH value of the reaction solution is 2, filter, the filtrate is layered, the toluene layer is discarded, and the water layer is Alkaline with 20% sodium hydroxide solution by weight to pH 12-13. Extract with chloroform 150ml×2, combine the extracts with NaSO 4 After drying, filtering, and evaporating to dryness, 27.22 g of light yellow oily substance 2-(7-methoxy-1-naphthyl)ethylamine was obtained, with a yield of 81%, MS (m / z)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com