Core-shell type magnetic surface imprinting nanometer composite material preparation method
A nanocomposite material, surface imprinting technology, applied in chemical instruments and methods, alkali metal compounds, alkali metal oxides/hydroxides, etc., can solve the problems of uniform coating and thickness control of difficult-to-polymerize layers, and achieve excellent regeneration performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
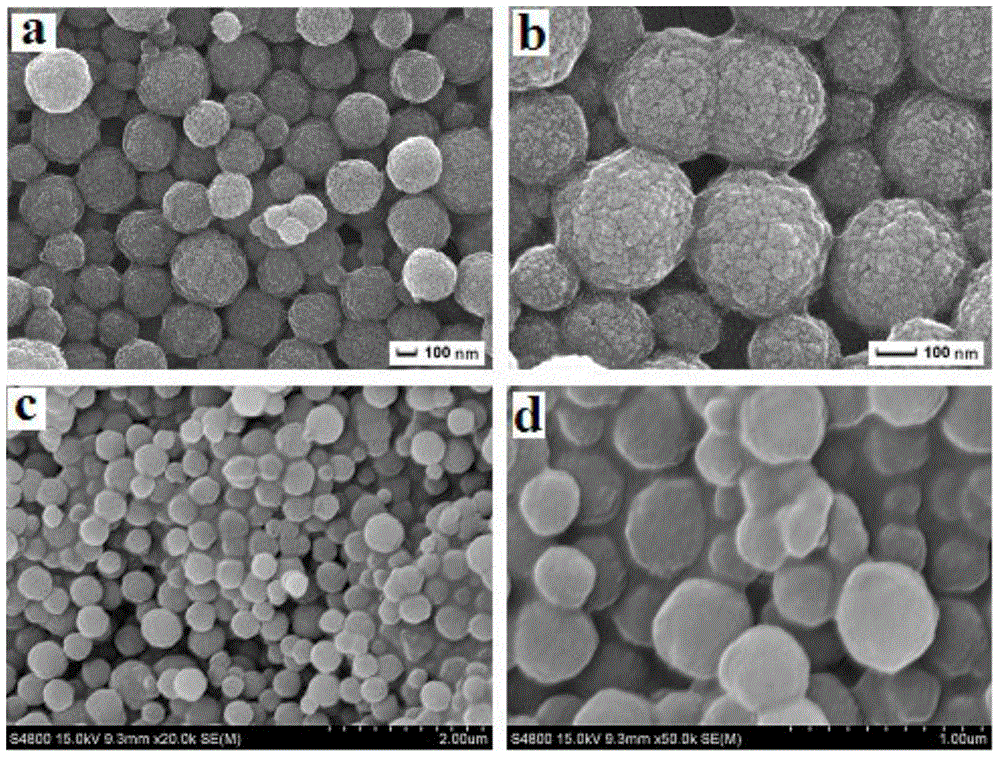
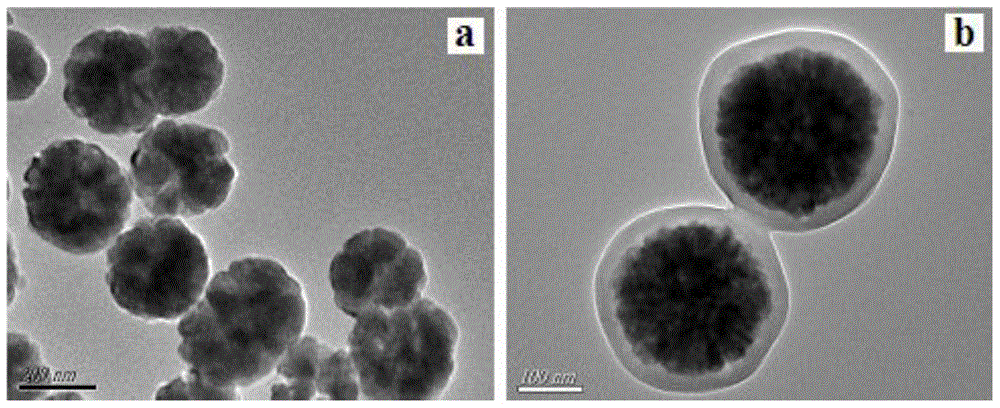
[0027] (1) Synthesis of Fe3O4 nanospheres
[0028] Ferric chloride hexahydrate (FeCl 3 ·6H 2 (2), wherein the volume and mass ratio of ethylene glycol and ferric chloride hexahydrate is middle 40: 1.0 (ml / g), vigorously stirred 30 min until forming uniform solution; In above-mentioned solution, add anhydrous sodium acetate ( NaAc) and polyethylene glycol (PEG-6000), wherein the mass ratio of anhydrous sodium acetate and polyethylene glycol is 3.0:0.5 (mg / mg) Stir vigorously until completely dissolved, then move into a stainless steel reactor, at 150 o C under reaction for 6.0 h, after the completion of the reaction, cool to room temperature, separate with rd-Fe-B permanent magnet, wash the product several times with absolute ethanol and deionized water, o C under vacuum drying, to obtain black magnetic ferric oxide nanospheres (Fe 3 o 4 ).
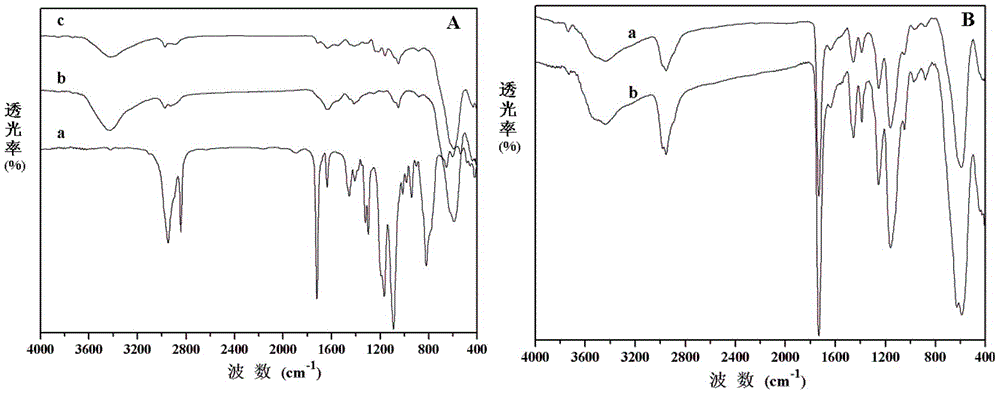
[0029] (2) Surface modification of Fe3O4 nanospheres
[0030] Add toluene, ferric oxide nanospheres and 3-(methacryloyloxy)prop...
Embodiment 2
[0034] (1) Synthesis of Fe3O4 nanospheres
[0035] Ferric chloride hexahydrate (FeCl 3 ·6H 2 (2), wherein the volume and mass ratio of ethylene glycol and ferric chloride hexahydrate is middle 40: 1.5 (ml / g), vigorously stirred 30 min until forming uniform solution; In above-mentioned solution, add anhydrous sodium acetate ( NaAc) and polyethylene glycol (PEG-6000), wherein the mass ratio of anhydrous sodium acetate and polyethylene glycol is 4.0:1.5 and stirred vigorously until completely dissolved, then moved into a stainless steel reaction kettle, at 220 o C under reaction for 12 h, after the completion of the reaction, cooled to room temperature, separated with rd-Fe-B permanent magnet, and washed the product with absolute ethanol and deionized water several times, at 60 o C under vacuum drying, to obtain black magnetic ferric oxide nanospheres (Fe 3 o 4 ).
[0036] (2) Surface modification of Fe3O4 nanospheres
[0037] Add toluene, ferric oxide nanospheres and 3...
experiment example 1
[0044] Experimental Example 1: Take 10 ml of tetracycline solutions with initial concentrations of 10, 30, 50, 80, 100, 150, and 200 μmol / L, respectively, and add them to centrifuge tubes, add 5.0 mg MMIPs and MNIPs respectively, and place the test solution at 298 After standing in a K water bath for 12.0 h, the supernatant was collected by magnetic separation, and the concentration of unadsorbed tetracycline molecules was measured with an ultraviolet-visible spectrophotometer, and the adsorption capacity was calculated based on the results. Figure 7 The results showed that with the increase of temperature and concentration, the adsorption capacity increased gradually, and finally reached the adsorption equilibrium, and the adsorption capacity of MMIPs to tetracycline was always greater than that of MNIPs, which proved that there were a large number of imprinted pores.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com