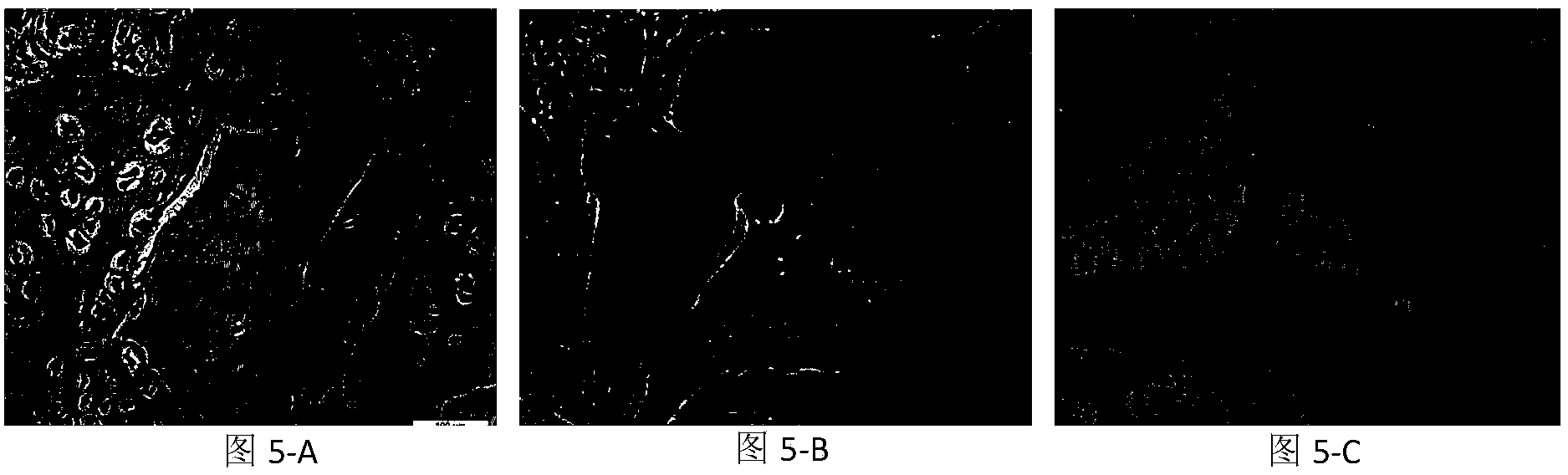
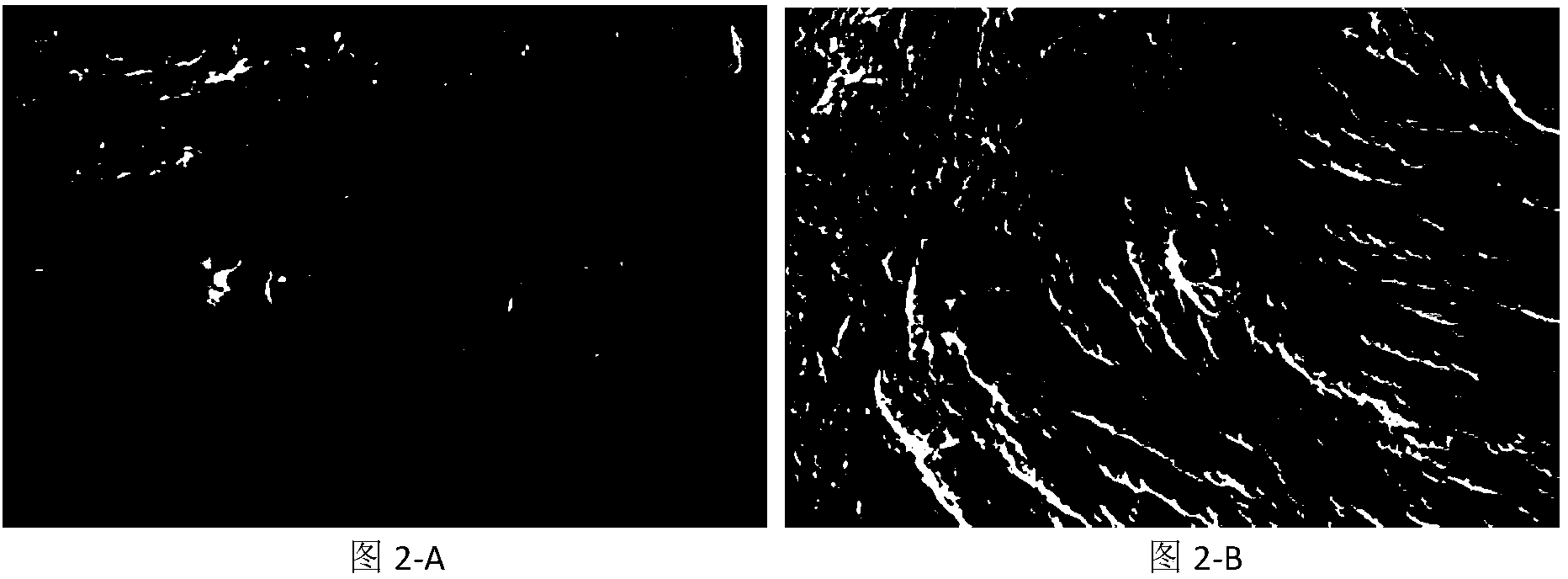
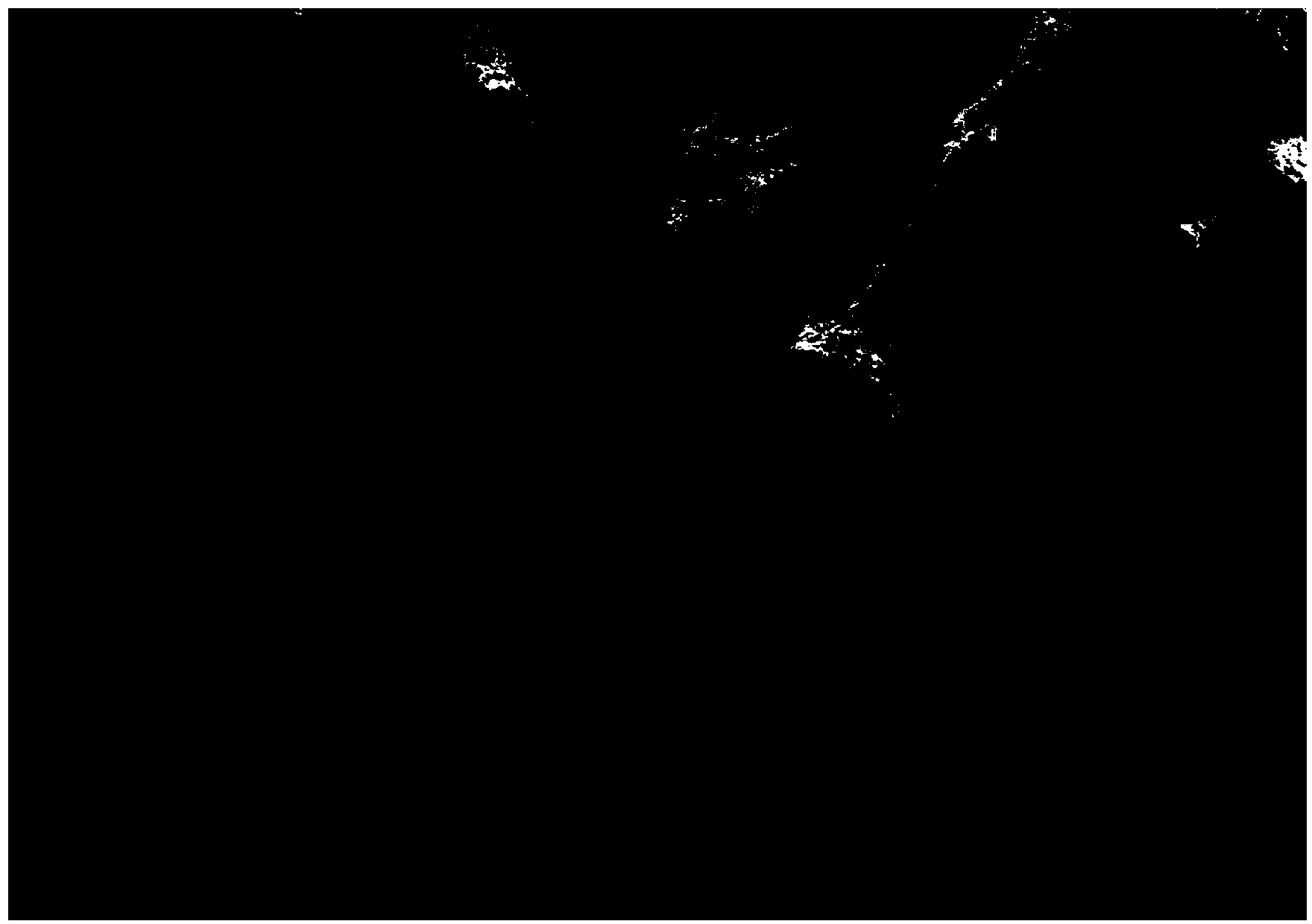
Antigen-removing biological bone and preparation method thereof
A deantigen and biological technology, applied in the field of biomaterials in tissue engineering, can solve the problems of inability to apply orthopedic defect repair, inability to effectively remove antigens, poor compressive performance of bone materials, etc., and achieve good capillary adsorption and retention of mechanical properties Features, the effect of strong pressure resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Preparation of deantigenated pork bone
[0038] Step 1. Bone block pretreatment: Take the metaphysis of pig femur and cut it into ≤1cm 3 After the small pieces, wash them with purified water to wash away excess bone marrow and blood, and then use a pulverizer to crush the bone pieces to 5mm-10mm.
[0039] Step 2. Partial deproteinization treatment: Put the bone particles obtained in Step 1 in a mixed solution of 0.5% SDS, 2% sodium hydroxide and 3% hydrogen peroxide; soak at 37°C for 1 hour, and then ultrasonically treat at 40KHz for 3 minutes After the bone particles are fully absorbed by the solution, they are shaken for 2 hours and washed with purified water until neutral. Taking this as a cycle, a total of 4 cycles were treated and then washed with purified water.
[0040] Step 3. Irradiation treatment: use 25-30KGy dose of Co 60 Process the bone particles obtained in step two.
[0041] Step 4. Organic matter removal: The bone particles obtained in...
Embodiment 2
[0045] Embodiment 2: Preparation of deantigenated bovine bone
[0046] Step 1. Pretreatment of bone fragments: cutting bovine femur or porcine femoral metaphysis into bone fragments, washing with purified water until there is no bone marrow and blood; crushing to a size of 5 mm to 10 mm.
[0047] Step 2. Partial deproteinization treatment: Put the bone particles obtained in Step 1 in a mixed solution of 2% Triton100-X, 1% sodium hydroxide and 2% hydrogen peroxide; soak at 37°C for 0.5 hours, and then undergo ultrasonic treatment at 40KHz After 3 minutes, the bone particles are fully absorbed by the solution, shaken for 1.5 hours, and washed with purified water until neutral. Take this as a cycle, and after a total of 4 cycles of treatment, the purified water is washed to neutral.
[0048] Step 3. Irradiation treatment: use 20KGy Co 60 The bone particles obtained in the second step are irradiated.
[0049] Step 4. Removal of organic matter: The bone particles obtained in Ste...
Embodiment 3
[0054] This embodiment is basically the same as Embodiment 1, the difference is:
[0055] Step 2 Partial deproteinization treatment: Put the bone particles obtained in Step 1 in a mixed solution of 1% Triton X-100, 2% sodium hydroxide and 3% hydrogen peroxide; soak at 37°C for 1 hour, and then undergo ultrasonic treatment at 40KHz After 3 minutes, after the bone particles have fully absorbed the solution, shake it for 1.5 hours, and wash it with purified water until neutral.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com