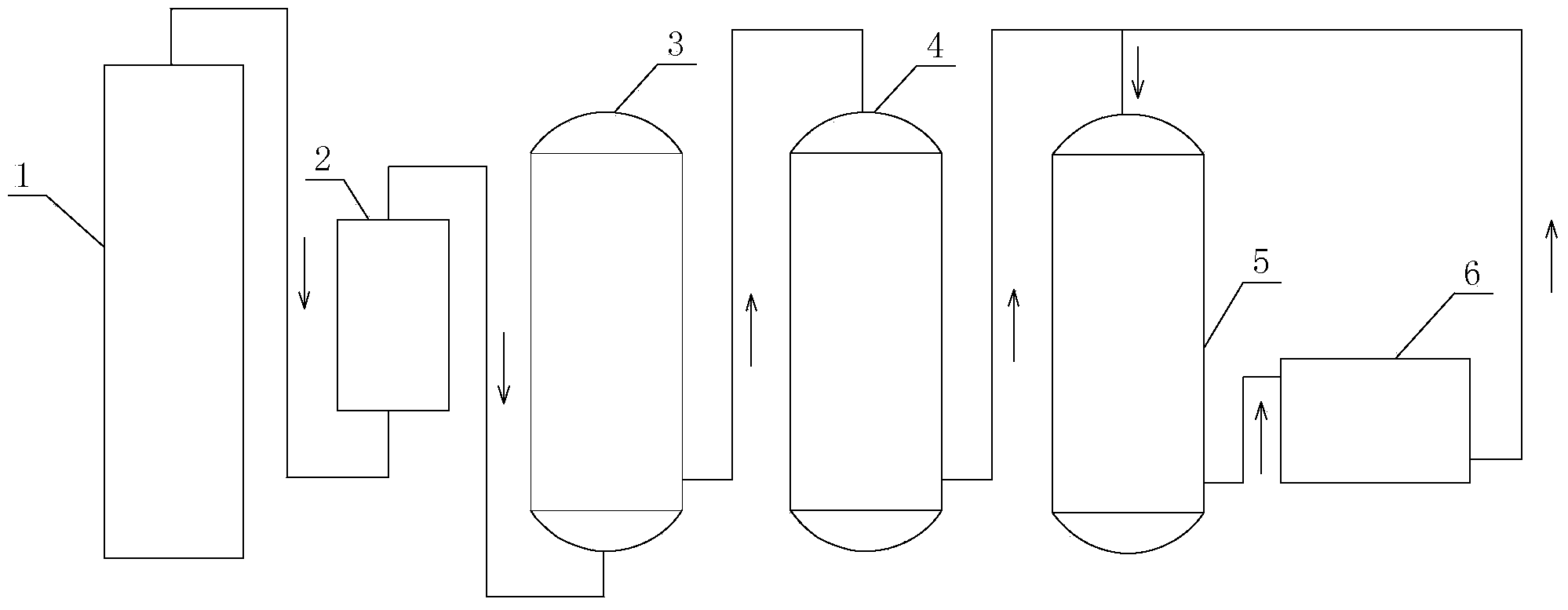
Preparation method of D,L-2-hydroxy-4-methylthiobutyric acid-trace element chelate
A technology of methylthiobutyric acid and methylthiobutyrate, which is applied in the field of chemical industry, can solve the problems of difficult trace metal element reaction, destruction of nutrient components, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The hydrocyanic acid mixed gas I from the hydrocyanic acid synthesis tower was detected. The composition of the hydrocyanic acid mixed gas I was: 8.87% hydrocyanic acid gas, 3.88% water vapor, 1.64% ammonia gas, 1.13% hydrogen gas, nitrogen gas 76.01%, oxygen 1.48%, carbon monoxide 5.67%, carbon dioxide 1.13%, methane 0.39%.
[0047] Hydrocyanic acid mixed gas I passes through a 75% sulfuric acid tower to absorb ammonia and water vapor in the mixed gas, and the composition of the obtained hydrocyanic acid mixed gas II is: 9.35% hydrocyanic acid gas, 1.57% hydrogen gas, and 79.44% nitrogen gas %, oxygen 1.71%, carbon monoxide 5.79%, carbon dioxide 1.50%, methane 0.64%.
Embodiment 2
[0049] Pass hydrogen cyanide mixed gas II into 223.3g of 94.5% methional with a mass fraction of 223.3g, which contains 3.3g of pyridine. React under normal pressure, control the reaction temperature to 45°C, the ventilation rate to 300L / min, absorb the tail gas with sodium hydroxide, and monitor the residual amount of methionaldehyde by HPLC. When the residual amount of methionaldehyde is less than 0.5%, it is the end of the reaction, and the feeding can be stopped. A total of 270.64 g of light yellow liquid was obtained, the content of 2-hydroxy-4-methylthiobutyronitrile was 98%, and the residual hydrocyanic acid was 0.5%.
Embodiment 3
[0051] Pass hydrogen cyanide mixed gas II into 223.3g of methional with a mass fraction of 94.5%, which contains 2.2g of pyridine and 10g of water. Under 0.15MPa, the reaction temperature is controlled to be 42°C, the ventilation rate is 280L / min, the tail gas is absorbed with sodium hydroxide, and the residual amount of methional is monitored by HPLC. When the residual amount of methionaldehyde is less than 0.5%, it is the end of the reaction, and the feeding can be stopped. A total of 279.54 g of light yellow liquid was obtained, the content of 2-hydroxy-4-methylthiobutyronitrile was 98%, and the residual hydrocyanic acid was 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com