Preparation method for celastrol derivatives, products and application thereof
A technology of tripterygne and its derivatives, which is applied in the field of preparation of tripteryne derivatives, and can solve the problems of low yield, few applications, and poor selectivity of chemical synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
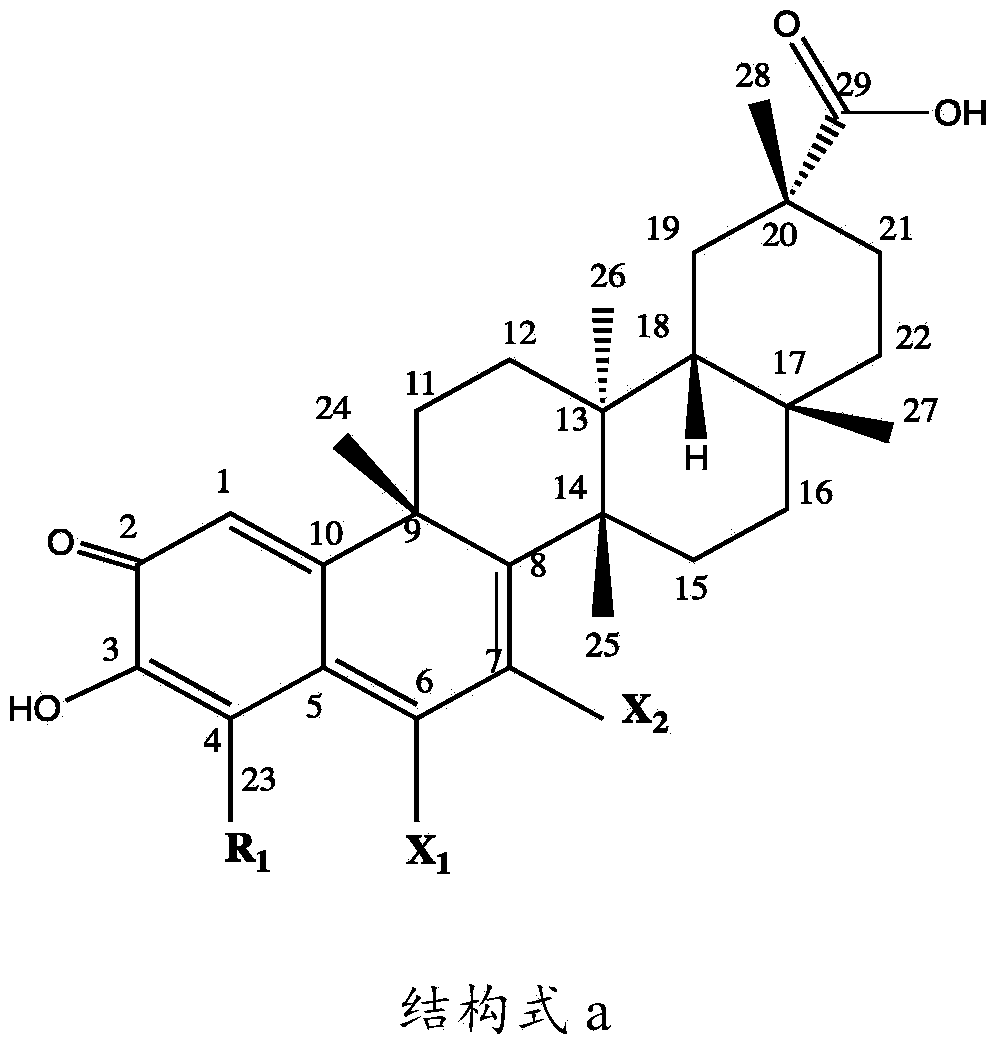
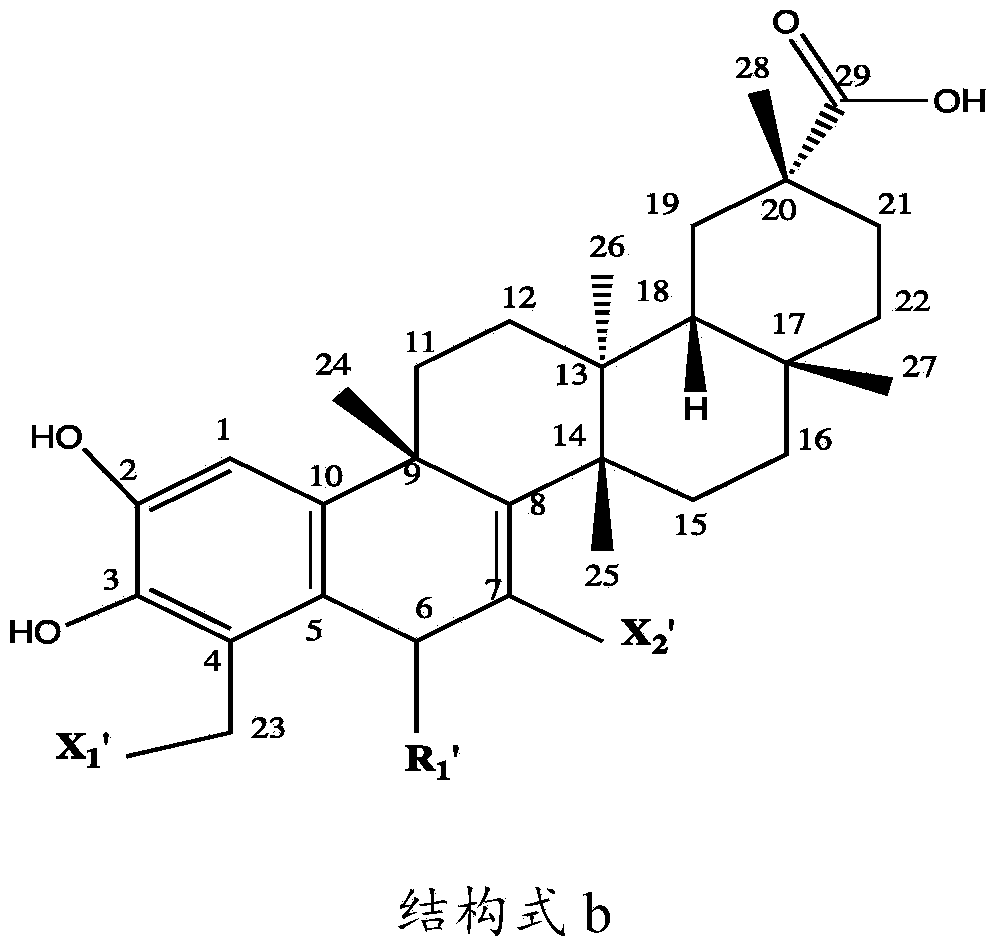
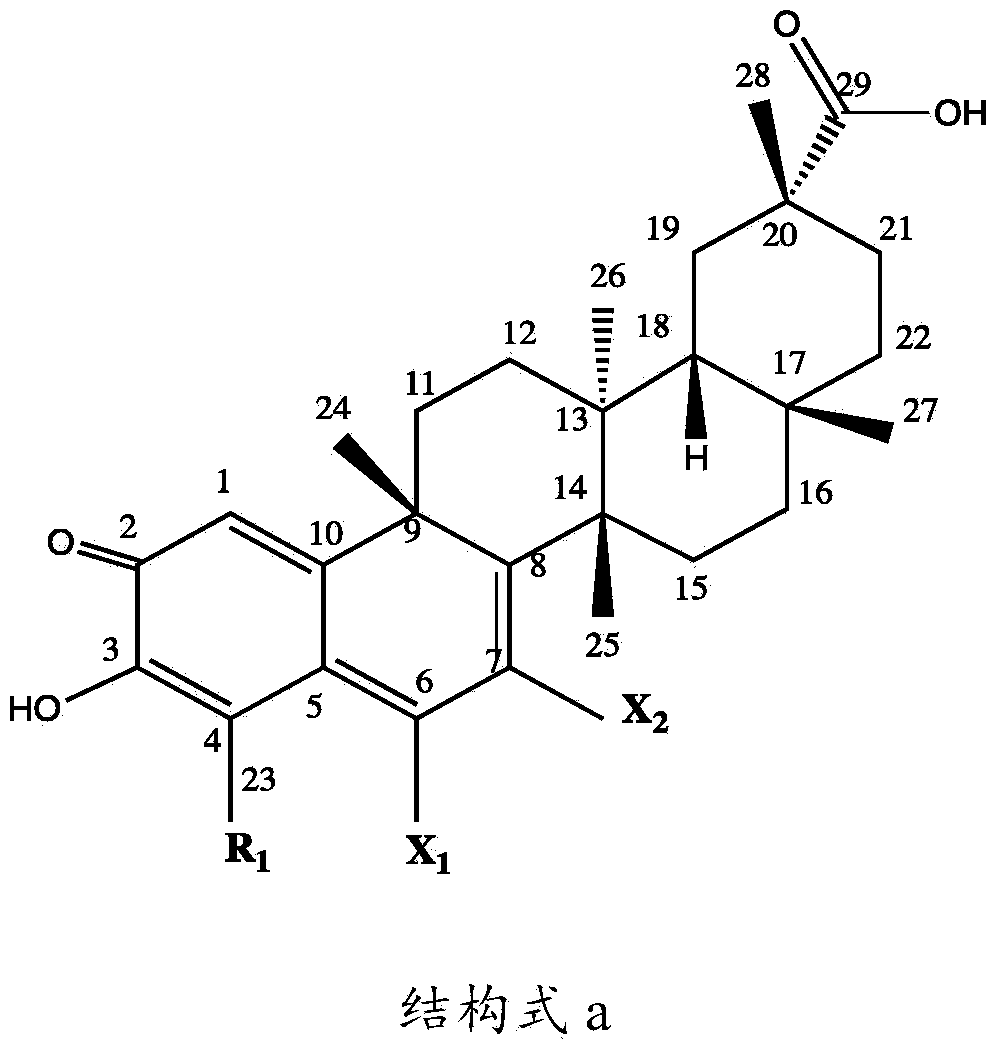
[0053] The present invention relates to a kind of preparation method of tripterine derivative, comprising the following steps:
[0054] Step 1: Take a transformed strain stored at 4°C and cultivated on a potato slant medium, place it in a constant temperature incubator at 25°C for 7 days, and blow off the spores on the slope with an appropriate amount of sterile water to make a single The concentration of the spore suspension was adjusted to 10 7 ~10 8 Individual / mL, acquired seed spore liquid;
[0055] Step 2: Inoculate the spore liquid obtained in Step 1 on the potato liquid medium with an inoculum amount of 100 mL or 5 mL, and cultivate it for 12 hours at a pH of 6.0, a temperature of 30°C ± 1°C, and a shaking speed of 200 r / min; press 0.1 ~4ug inoculation amount, add various combined elicitors in different proportions, continue to cultivate for 12 hours, then add tripterycin at an inoculation amount of 1~10ug, and then continue to culture for 5 days, detect various tript...
Embodiment 1
[0094] Example 1: Preparation of tripterine derivatives
[0095] 1, Preparation of 6-hydrotripterine
[0096] Inoculate 5 mL (10 8Cunninghamella blakesleana (Cunninghamella blakesleana) AS3.970 spore liquid, cultivated for 12 hours at a pH of 6.0, at a temperature of 30°C±1°C, and at a shaking speed of 200r / min; inoculum size of 0.2ug , adding methyl jasmonate (MeJA, 0.78mmol / mL), betamethasone valerate (5mg / L) and carboxymethyl-β-cyclodextrin ( BCD, 1.79mmol / mL), after continuing to cultivate for 12h, add tripterine according to the inoculum amount of 5%, then continue to cultivate for 5 days, stop the cultivation, then centrifuge the filtrate at 8000r / min for 10min to remove the precipitate, the filtrate Extract with an equal volume of ethyl acetate for 3 times, evaporate the extract to dryness, dissolve it with 2 mL of acetone, and then separate it with a chromatographic silica gel column. Elution is clean, and then successively use petroleum ether: dichloromethane eluen...
Embodiment 2
[0140] Embodiment two, pharmacodynamics evaluation experimental example
[0141] In the following experimental examples, the test sample is provided by the preparation method embodiment of the present invention, and the precursor compound tripterine is used as a positive control.
[0142] 1. The growth inhibitory effect of 8 compounds of the present invention on human lung cancer cell A549 cells cultured in vitro
[0143] Methods: Human A549 lung cancer cells were cultured in DMEM medium (Gibco, USA) containing 10% fetal bovine serum at 37°C, 5% CO 2 , tumor cells 0.7x10 4 / well was inoculated in a 96-well plate, and after 24 hours, the compound diluted with dimethyl sulfoxide (200uM) and PBS solution was added to make the final concentration of the culture medium 10 -4 、10 -5 、10 -6 、10 -7 、10 -8 M, after 72 hours of treatment, discard the culture medium, fix the cells with 10% cold trichloroacetic acid, stain with sulforhodamine B (SRB) solution, wash away the unbound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com