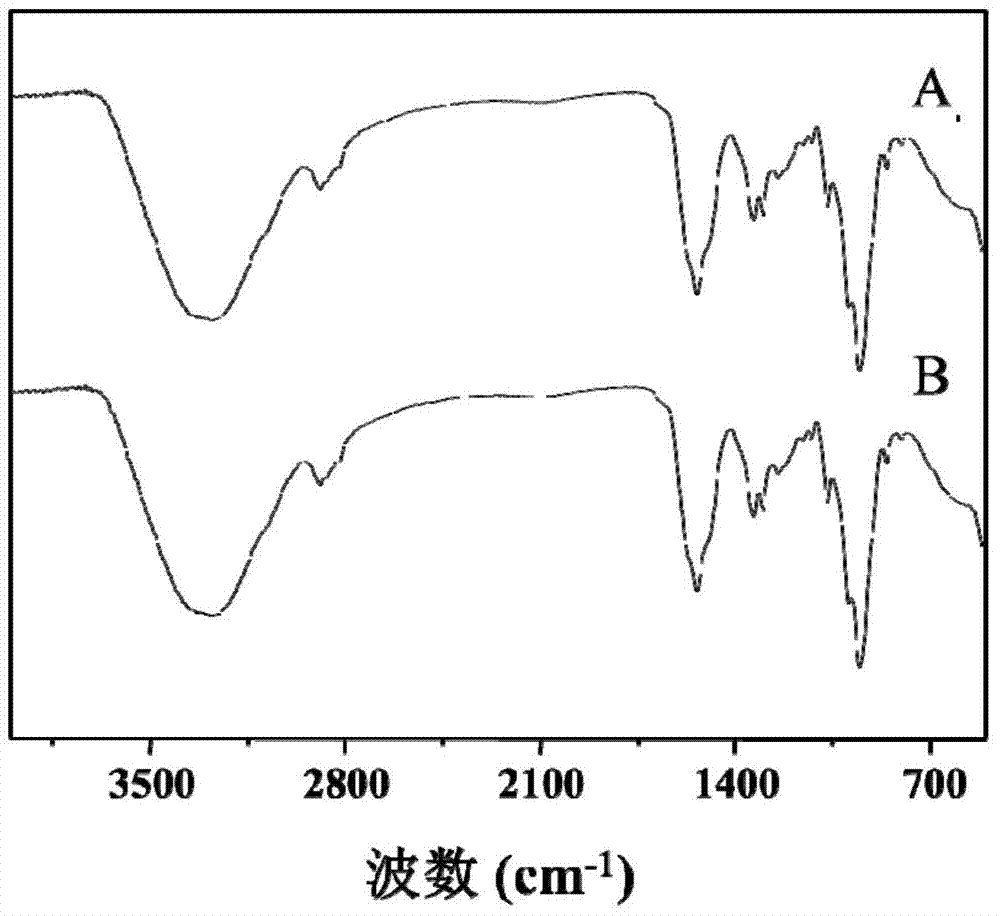
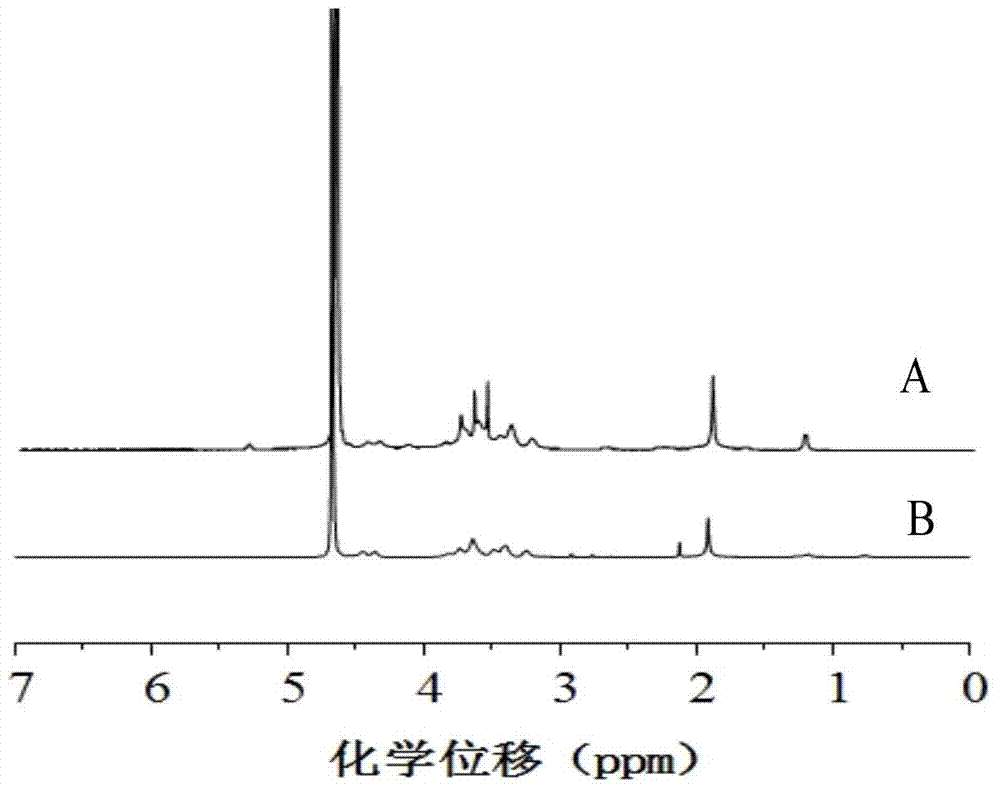
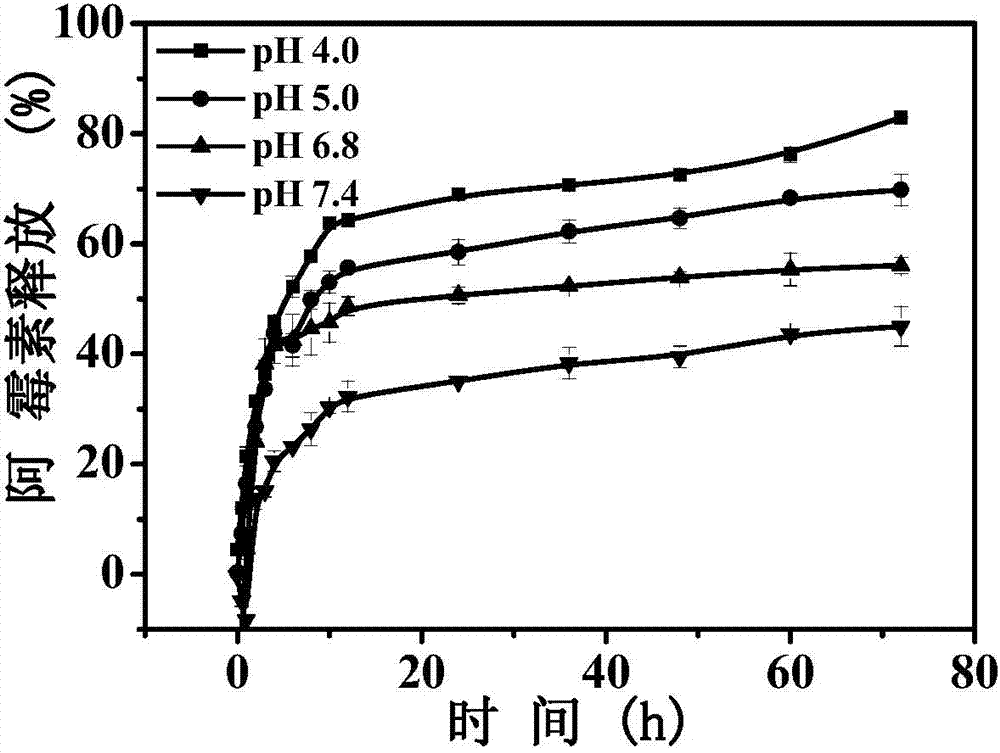
Hyaluronic acid-adriamycin bonding medicine and preparation method thereof
A technology of hyaluronic acid and doxorubicin hydrochloride, which is used in pharmaceutical formulations, anti-infective drugs, anti-tumor drugs, etc., can solve the problems of lack of doxorubicin release, poor biocompatibility, and low drug loading, and achieve good Biological activity and biocompatibility, stable performance, enhanced efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention also provides a preparation method of a hyaluronic acid-doxorubicin bonded drug, comprising:
[0031] mixing and reacting the buffer solution in which doxorubicin hydrochloride is dissolved with hyaluronic acid to obtain a hyaluronic acid-doxorubicin bonded drug having a structure of formula (I);
[0032] Alternatively, the buffer solution in which doxorubicin hydrochloride is dissolved is mixed with hyaluronic acid and sodium cyanoborohydride and reacted to obtain a hyaluronic acid-doxorubicin bonded drug having a structure of formula (II).
[0033] The hyaluronic acid-doxorubicin bonded drug with the structure of formula (I) is prepared according to the following method:
[0034] First, doxorubicin hydrochloride is dissolved in a buffer solution to obtain a mixed solution; the doxorubicin hydrochloride has the structure shown in formula (III):
[0035]
[0036] The buffer solution is preferably an acetate buffer solution, specifically a sodiu...
Embodiment 1~8
[0055] Prepare an acetate buffer solution with pH=5, take 5 mL of the prepared buffer solution and place them in 8 round-bottomed flasks respectively, add 0.05 g of doxorubicin hydrochloride to each flask, stir and dissolve in the dark, and then According to the dosage ratio in Table 1, add hyaluronic acid and sodium cyanoborohydride to each flask, the number average molecular weight of the hyaluronic acid is 2483g.mol -1 , then seal each reaction bottle, according to the reaction conditions in Table 1, react in the dark for 52h, after the reaction, adjust the pH of each reaction solution to 7.4 with sodium bicarbonate solution, then use a dialysis bag with a molecular weight cut-off of 7000Dalton for dialysis for 48h, filter Freeze-dried to obtain hyaluronic acid-doxorubicin bonded drug respectively, the experimental results are shown in Table 1, Table 1 is the reaction conditions and yields of the hyaluronic acid-doxorubicin bonded drug prepared in Examples 1-8 of the present...
Embodiment 9~16
[0061] Prepare an acetate buffer solution with pH=5, take 5 mL of the prepared buffer solution and place them in 8 round-bottomed flasks respectively, add doxorubicin hydrochloride to each flask according to the ratio in Table 2, and keep away from light. After stirring and dissolving, add hyaluronic acid and sodium cyanoborohydride to each flask according to the dosage ratio in Table 2, the number average molecular weight of the hyaluronic acid is 2483g.mol -1 , then seal each reaction bottle, and react in the dark at 40°C for 52h. After the reaction, adjust the pH of each reaction solution to 7.4 with sodium bicarbonate solution, then dialyze with a dialysis bag with a molecular weight cut-off of 7000Dalton for 48h, filter and freeze-dry to obtain Hyaluronic acid-doxorubicin conjugated drug, the experimental results are shown in Table 2, Table 2 is the reaction conditions and yields of the hyaluronic acid-doxorubicin conjugated drug prepared in Examples 9-16 of the present in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com