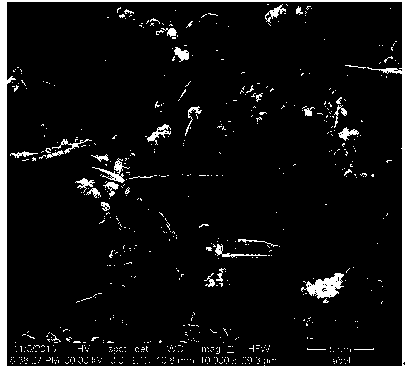
Method for preparing arborization fine copper powder in electrolysis mode
A dendritic and fine technology, applied in the field of electrolytic preparation of dendritic fine copper powder, can solve the problems of complex electrolyte, complex raw materials, numerous steps, etc., and achieve the effects of avoiding hydrogen precipitation, simple preparation and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
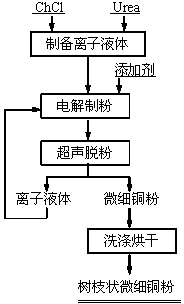
[0022] Such as figure 1 Shown, this electrolysis prepares the method for dendritic fine copper powder, and its concrete steps are as follows:
[0023] (1) First, choline chloride and urea are uniformly mixed at a molar ratio of 1:2 to prepare a deep eutectic solvent-based ionic liquid, and then add additives to prepare an electrolyte, wherein the additives are polyvinyl alcohol and polyvinyl alcohol at a mass ratio of 1:1. Vinylpyrrolidone mixture, the addition amount is 10g / L;
[0024] (2) Put pure copper as the anode and titanium sheet as the cathode in the electrolyte obtained in step (1). The area ratio of the cathode to the anode is 1:1, and the distance between the electrodes is 10mm. The current density is 10A / m 2 Under the condition of electrolysis for 30 minutes, dendritic fine copper powder is obtained on the cathode, wherein the pure copper anode is the standard cathode copper produced by electrolytic refining, the cathode material of the titanium sheet is TA1, an...
Embodiment 2
[0028] Such as figure 1 Shown, this electrolysis prepares the method for dendritic fine copper powder, and its concrete steps are as follows:
[0029] (1) Firstly, choline chloride and urea were uniformly mixed at a molar ratio of 1:2 to prepare a deep eutectic solvent-based ionic liquid, and then an additive was added to prepare an electrolyte. The additive was oxalic acid, and the addition amount was 30g / L;
[0030] (2) Put pure copper as the anode and titanium sheet as the cathode in the electrolyte obtained in step (1). The area ratio of the cathode to the anode is 1:2, and the distance between the electrodes is 40mm. The current density is 300A / m 2 Under the conditions of electrolysis for 120 minutes, dendritic fine copper powder is obtained on the cathode, wherein the pure copper anode is high-purity cathode copper produced by electrolytic refining, the cathode material of titanium sheet is TA2, and the stirring speed is 700r / min;
[0031] (3) The dendritic fine copp...
Embodiment 3
[0034] Such as figure 1 Shown, this electrolysis prepares the method for dendritic fine copper powder, and its concrete steps are as follows:
[0035] (1) Firstly, choline chloride and urea were uniformly mixed at a molar ratio of 1:2 to prepare a deep eutectic solvent-based ionic liquid, and then an additive was added to prepare an electrolyte, wherein the additive was ethylenediaminetetraacetic acid, and the amount added was 20g / L;
[0036] (2) Place pure copper as the anode and titanium sheet as the cathode in the electrolyte obtained in step (1). The area ratio of the cathode to the anode is 1:1.5, and the distance between the electrodes is 30mm. The current density is 200A / m 2Under the conditions of electrolysis for 90 minutes, dendritic fine copper powder is obtained on the cathode, wherein the pure copper anode is high-purity cathode copper produced by electrolytic refining, the material of the titanium sheet cathode is TA1, and the stirring speed is 600r / min;
[003...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com