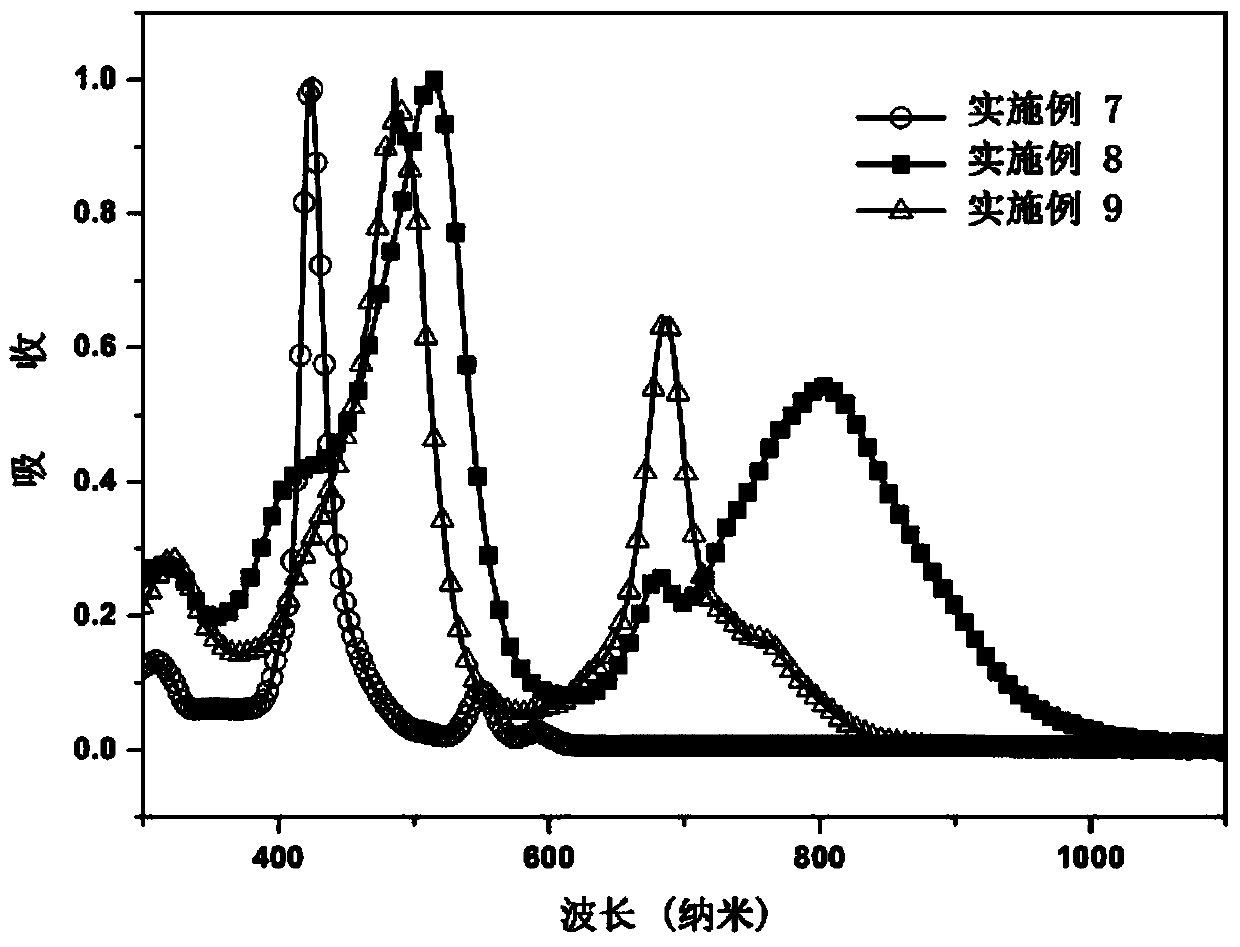
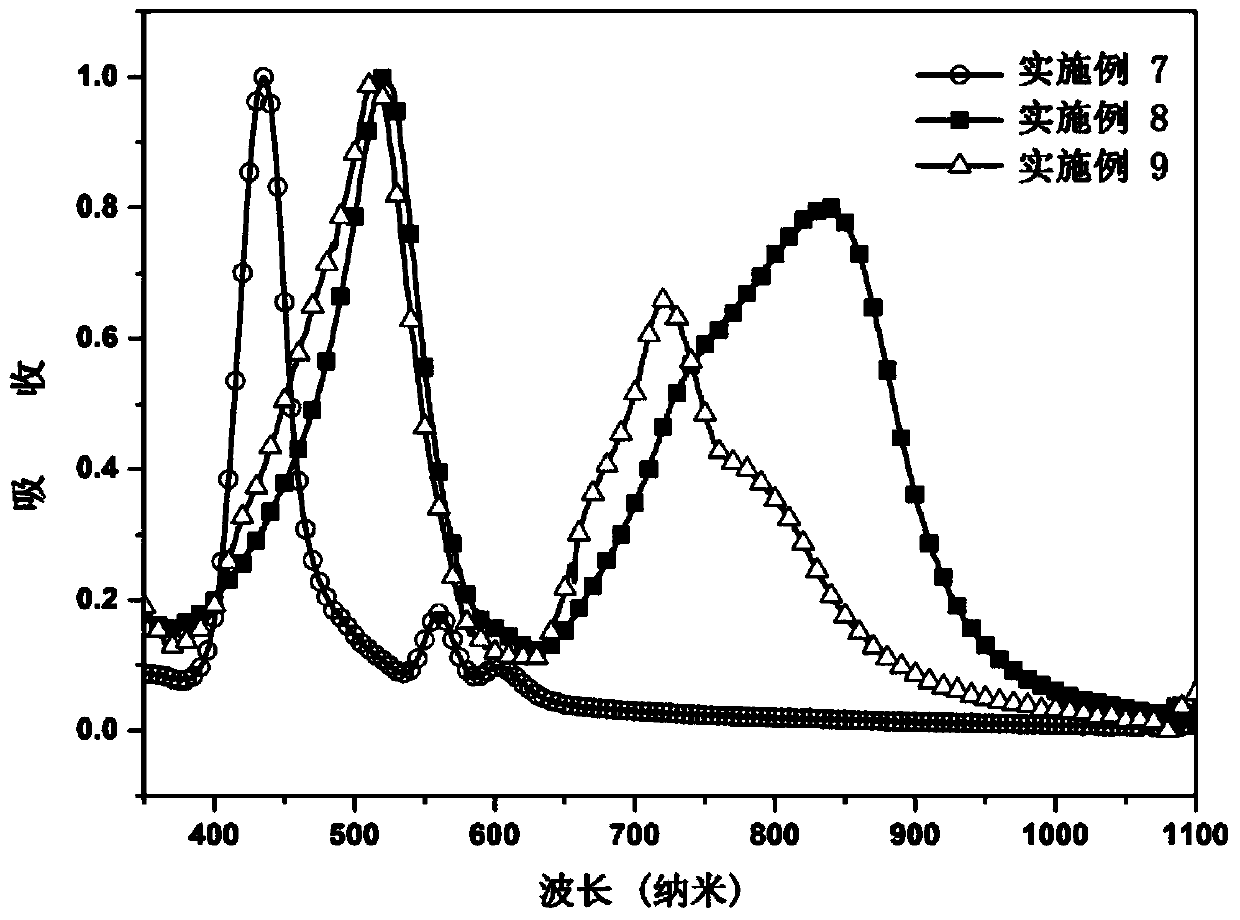
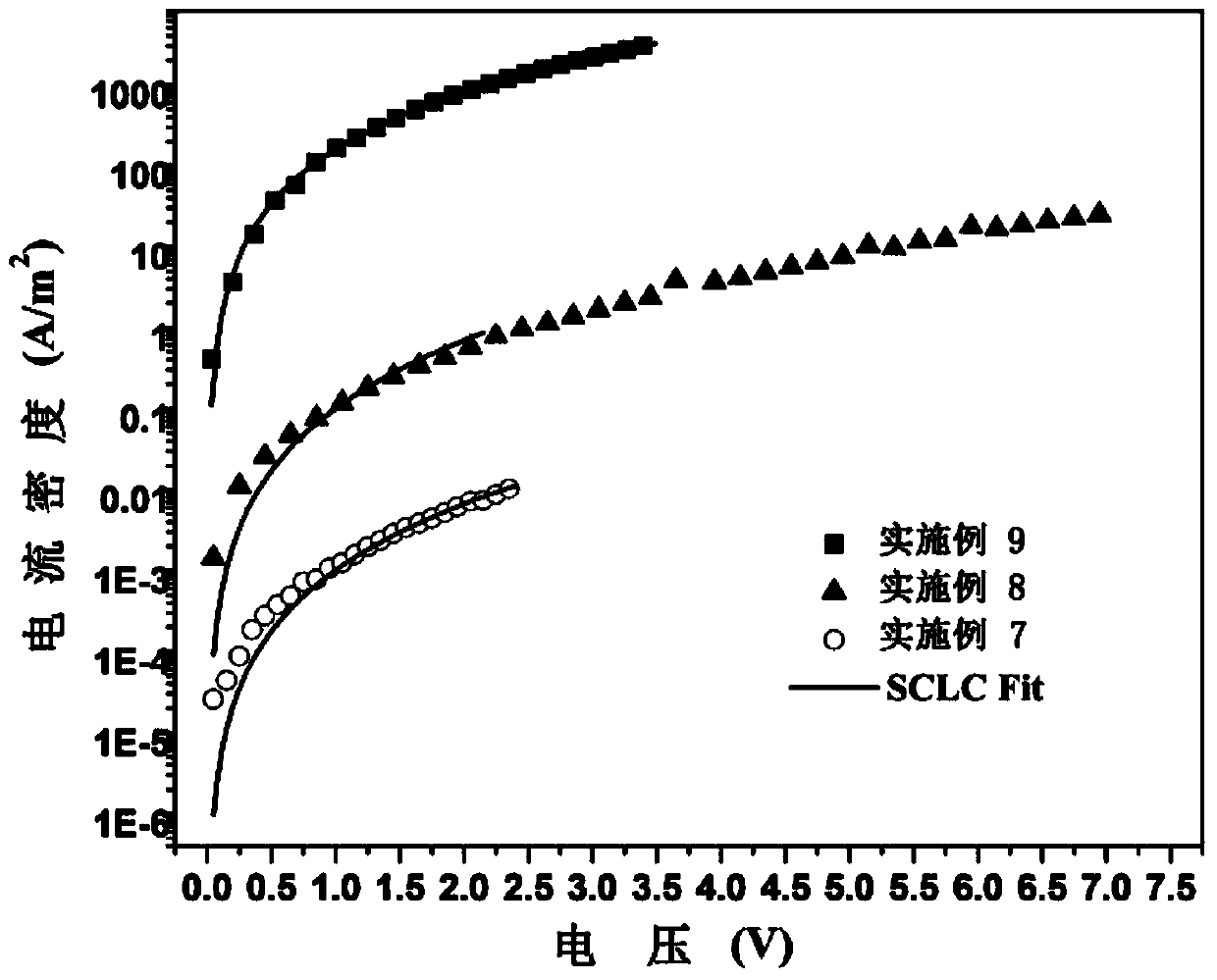
Porphyrin organic small molecular photovoltaic material and preparation method thereof
A photovoltaic material and small molecule technology, applied in photovoltaic power generation, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problems of low photoelectric conversion efficiency of organic solar cells, improve carrier mobility, optimize solubility, increase Effects of large π conjugated systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of 5,15-bis(3,5-bis(dodecyloxy)benzene)porphyrin
[0037]
[0038] In a 1000mL two-neck round bottom flask, add 3,5-bis(dodecyloxy)benzaldehyde (2.304g, 4.86mmol), bipyrromethane (700mg, 4.86mmol) and 500mL of dichloromethane, and ventilate with nitrogen 30 minutes, then add 0.25mL of trifluoroacetic acid, stir the reaction at room temperature for 12 hours, then add 1.8g of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), continue stirring After reacting for 12 hours, 5 mL of triethylamine was added to quench the reaction. After the reaction, the crude product was obtained by silica gel / (dichloromethane as eluent) column chromatography and spin-dried, and then recrystallized by chloroform / methanol to obtain a dark red solid. 1 H NMR (300MHz, CDCl 3 ):δ10.30(s,2H),9.39(d,J=4.6Hz,4H),9.18(d,J=4.6Hz,4H),7.42(s,4H),6.91(s,2H),4.15 (t,J=6.6Hz,8H),1.93(m,8H),1.47(m,8H),1.24(m,64H),0.83(m,12H),-3.2(s,2H). 13 C NMR (75MHz, CDCl 3 ):δ158.67,147.02,145.31,143.16...
Embodiment 2
[0040] Synthesis of 5,15-bisbromo-10,20-bis(3,5-bis(dodecyloxy)phenyl)zinc porphyrin
[0041]
[0042] Dissolve 5,15-bis(3,5-bis(dodecyloxy)benzene)porphyrin (840mg, 0.7mmol) in 700mL of chloroform, add 35mL of pyridine, avoid light, and then add bromobutylene Imide (NBS) (274mg, 1.54mmol), reacted at 0°C for 30 minutes, then continued to react at room temperature for 4 hours, and finally quenched the reaction with acetone. After the reaction is complete, add water, extract with chloroform, dry over anhydrous sodium sulfate, spin dry the solvent and dissolve in 50mL of chloroform solution, then add 12mL of zinc acetate methanol solution (243mg, 1.2mmol of zinc acetate dissolved in 12mL of methanol solvent ), and reflux for 2 hours in the dark. After the reaction was completed, it was washed with water, dried with anhydrous sodium sulfate, spin-dried to dry the solvent, and purified by silica gel column chromatography to obtain a bright red solid. 1H NMR (300MHz, CDCl 3 )...
Embodiment 3
[0044] Synthesis of 5,15-bis(trimethylsilylacetylene)-10,20-bis(3,5-bis(dodecyloxy)phenyl)zinc porphyrin
[0045]
[0046] In a 100mL two-neck round-bottomed flask, add 5,15-bisbromo-10,20-bis(3,5-bis(dodecyloxy)benzene)zinc porphyrin (710mg, 0.5mmol), 25mL tetrahydrofuran and 12.5mL triethylamine, nitrogen gas for 30 minutes, then added bis (triphenylphosphine) palladium dichloride (17.5mg, 0.025mmol), copper iodide (CuI) (5mg, o.o25mmol) and trimethyl Silicon acetylene (200mg, 2mmol), protected from light, the reaction was stirred at room temperature for three days. After the reaction was completed, it was washed with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and then subjected to column chromatography on silica gel / (dichloromethane / petroleum ether=1:1 as eluent), and spin-dried to obtain a green solid. 1 H NMR (300MHz, CDCl 3 ):δ9.68(d,J=4.6Hz,4H),9.04(d,J=4.6Hz,4H),7.33(s,4H),6.89(s,2H),4.10(t,J=6.4Hz ,8H),1.82(m,8H),1.50(m,8H),1.23(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com