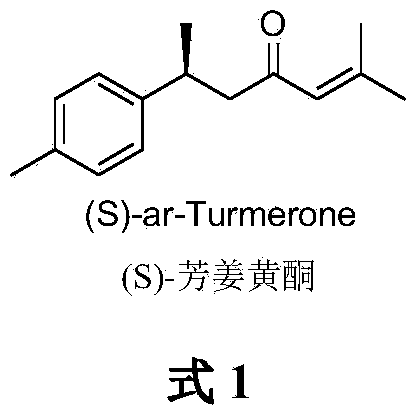
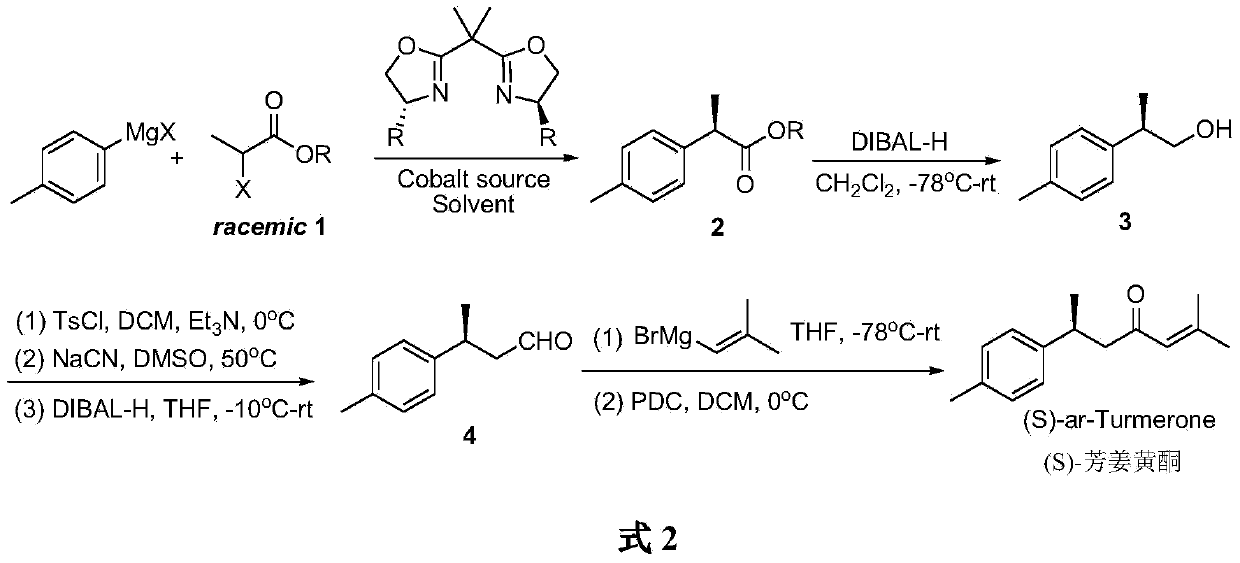
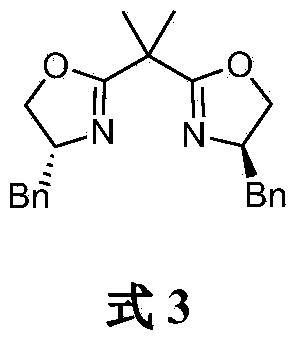
Novel method for asymmetrically catalyzing (S)-arturmerone
A synthetic method, the technology of aromatic curcumone, applied in the field of new asymmetric catalytic synthesis-aromatic curcumone, can solve the problems of cumbersome reaction steps, harsh reaction conditions, a large number of chiral source reagents, etc. The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of (R)-benzyl p-toluene propionate 2
[0023] Under the protection of argon, add CoI in a dry Schlenk bottle 2 (187.2mg, 0.6mmol), after vacuum drying for 2h, add anhydrous tetrahydrofuran (18mL) and bisoxazoline chiral ligand L1 (261.0mg, 0.72mmol), and stir at room temperature for 1h. Add racemic 2-bromophenylpropionate (1.45g, 6mmol) to the mixture, lower the temperature of the reaction solution to -80°C, add p-methylmagnesium bromide (12mL, 1.0M THF solution, 12mmol) dropwise ). Stirring was continued at -80°C for 12 h, and the reaction was quenched by adding saturated ammonium chloride aqueous solution. The reaction solution was extracted with ether, the organic layers were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure and purified by silica gel column chromatography (n-hexane / ethyl acetate 40:1) to obtain (R)-p-toluenepropionic acid as a colorless oil Benzyl ester 2 (1.34 g, 88% yield, 93% optical purity). [α] D ...
Embodiment 2
[0027] Synthesis of (R)-p-tolylpropanol 3
[0028] Under the protection of argon, the dichloromethane (60mL) solution dissolved with (R)-benzyl p-toluene propionate 2 (1.27g, 5mmol) was lowered to -78°C, and diisobutylaluminum hydride ( DIBAL-H) (12mL, 1.0M toluene solution, 12mmol), warmed up to room temperature and continued to stir the reaction until no raw material was detected by TLC. The reaction was quenched with potassium sodium tartrate aqueous solution (60mL), and continued to stir for 6h. The organic phase was separated and the aqueous phase was extracted with dichloromethane. The organic layers were combined, washed with water, dried over anhydrous sodium sulfate, concentrated under reduced pressure and purified by silica gel column chromatography (n-hexane / ethyl acetate 4:1) to obtain a colorless oil (R)-p-tolylpropanol 3(0.71 g, yield 94%, optical purity 92%). [α] D 20 =+15.9(c1.1,CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ:7.16–7.10(m,4H),3.65(d,J=6.9Hz,2H),2....
Embodiment 3
[0030] Synthesis of (S)-p-tolylbutyraldehyde 4
[0031] Under argon protection, (R)-p-tolylpropanol 3 (0.6g, 4mmol) was dissolved in dichloromethane (16mL), and triethylamine (0.81g, 1.2mL, 8mmol) and p-toluenesulfonate were added at 0°C Acid chloride (0.9g, 0.76mmol), after stirring for 6 hours, with NH 4 Cl aqueous solution was used to quench the reaction, the aqueous phase was extracted with diethyl ether, the organic phases were combined and washed with water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude product of sulfonate of p-tolylpropanol. The crude sulfonate was dissolved in DMSO (8 mL), NaCN (0.39 g, 8 mmol) was added, and the reaction was stirred at 50 °C for 1 h. The reaction solution was diluted with water, and the aqueous phase was extracted with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product of p-toluen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com