Febuxostat intermediate and preparation method thereof
A technology for febuxostat and intermediates, which is applied in the field of new febuxostat intermediates and their preparation, can solve the problems of long operation cycle, low product purity, unfavorable production operation and the like, and achieves ideal yield and high purity , and good quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] C. The preparation method of febuxostat
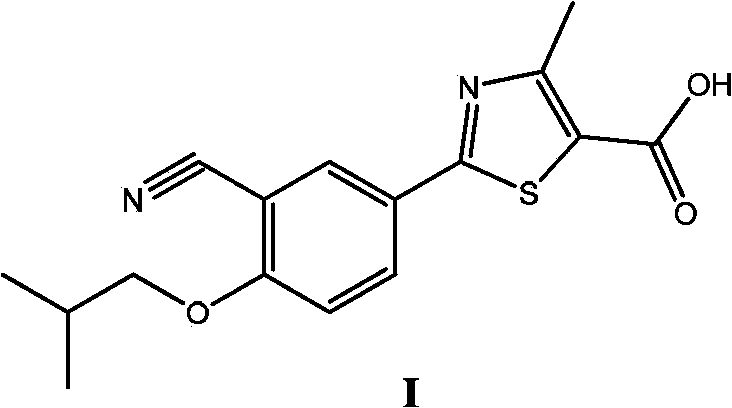
[0091] The present invention also provides a method for preparing Febuxostat, wherein said Febuxostat is shown in formula I:
[0092]
[0093] Described method comprises the following steps:
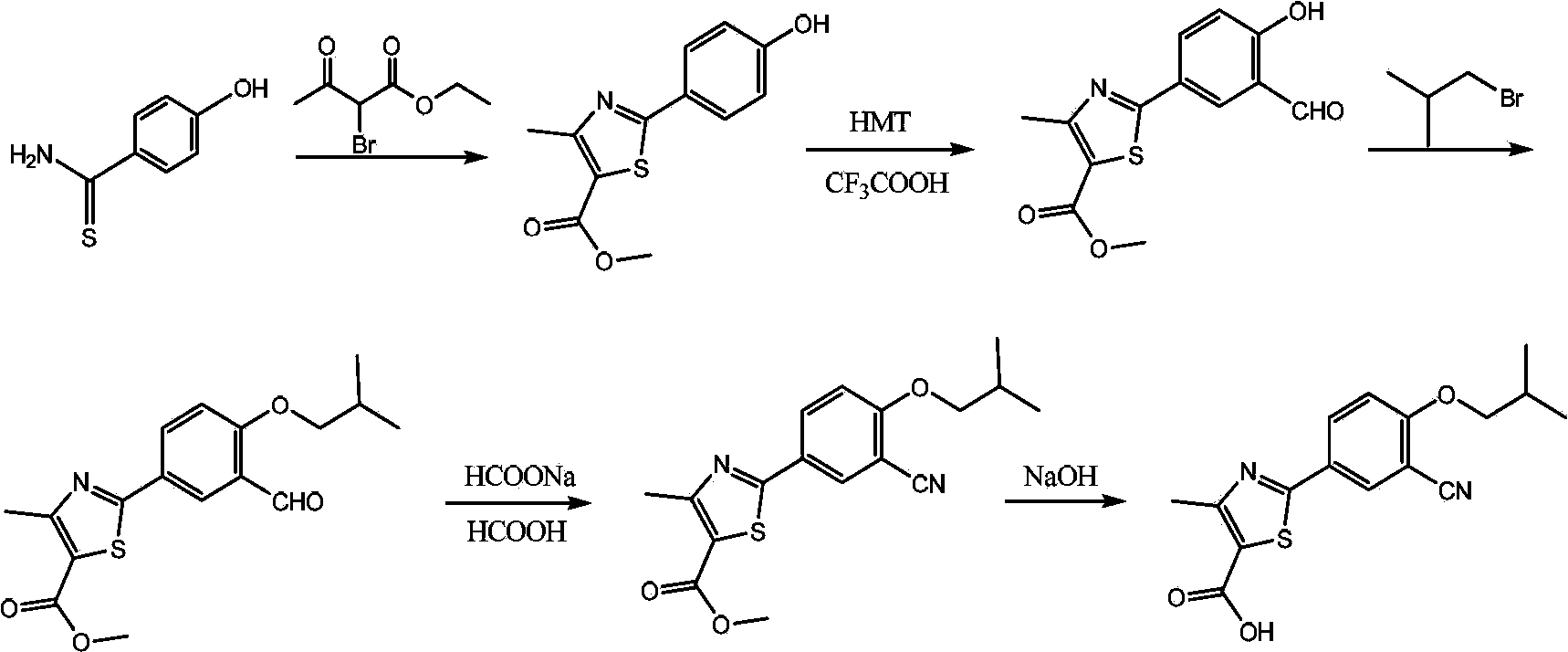
[0094] Make the compound shown in formula V carry out the hydrolysis reaction shown below in the presence of hydrolysis catalyst, to obtain described Febuxostat:
[0095]
[0096] Preferably, the molar ratio of the hydrolysis catalyst to the compound represented by formula V is (1-10):1; more preferably (1-5):1. If the molar ratio of the hydrolysis catalyst to the compound represented by formula V is too high, the raw material cost will be increased; if it is too low, the hydrolysis reaction will be incomplete.
[0097] Preferably, the hydrolysis catalyst is an alkali; preferably, the alkali is selected from sodium hydroxide or potassium hydroxide.
[0098] Preferably, the reaction temperature of the hydrolysis reaction is 50°C to...
Embodiment 1
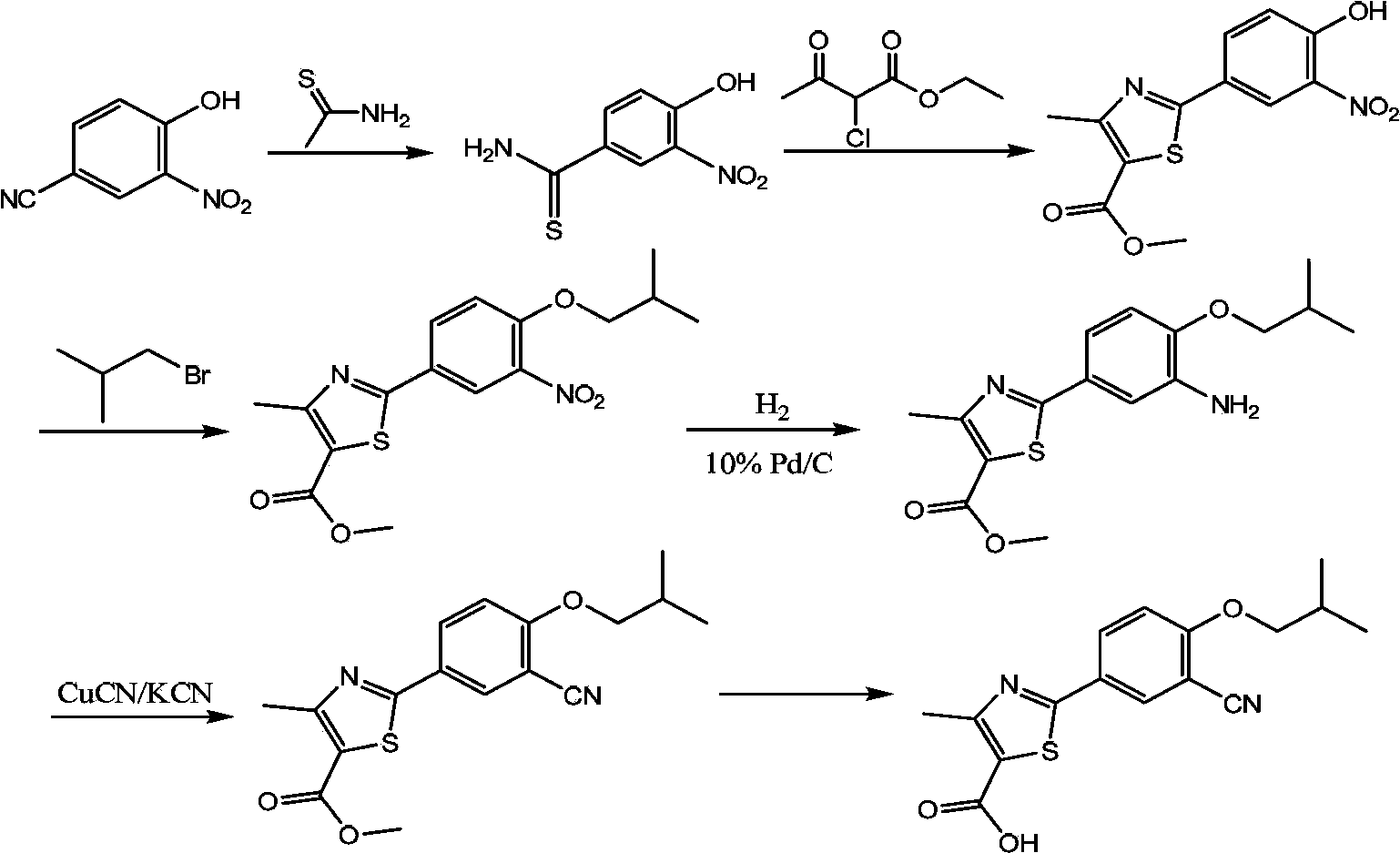
[0124] Embodiment 1: the preparation of the Grignard reagent shown in formula III:
[0125] Keep warm at about 30°C, pass nitrogen protection, add 200ml tetrahydrofuran (hereinafter referred to as THF), 72.9g (3.00mol) of pretreated magnesium chips, and 12.7g (0.05mol) of iodine into a 1000ml reaction bottle, and stir for 1 After 1 hour, the temperature was raised to 45°C, and at the same time, a mixture of 680.4g (2.50mol) of 5-bromo-2-isobutoxybenzamide and 12.7g (0.05mol) of iodine dissolved in 250ml THF was slowly added dropwise. React at 45°C for 5 hours, cool to 15°C to 20°C, and set aside.
[0126] Among them, the pretreatment method of magnesium chips is as follows: stir and wash with 5% hydrochloric acid for 30 minutes, quickly filter and rinse with acetone (minimize the time of contact with air), and use it immediately after vacuum drying.
Embodiment 2
[0127] Embodiment 2: the preparation of the Grignard reagent shown in formula III:
[0128] Keep warm at about 20°C, pass nitrogen protection, add 200ml methyl tert-butyl ether, 72.9g (3.00mol) of pretreated magnesium chips, and 12.7g (0.05mol) of iodine into a 1000ml reaction bottle, and stir for 1 hour , the temperature was raised to 50°C, and at the same time, a mixture of 680.4 g (2.50 mol) of 5-bromo-2-isobutoxybenzamide dissolved in 300 ml of methyl tert-butyl ether and 12.7 g (0.05 mol) of iodine was slowly added dropwise, After dropping, keep warm at 45°C and react for 6 hours, cool to 15°C to 20°C, and set aside.
[0129] Among them, the pretreatment method of magnesium chips is as follows: stir and wash with 5% hydrochloric acid for 30 minutes, quickly filter and rinse with acetone (minimize the time of contact with air), and use it immediately after vacuum drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com