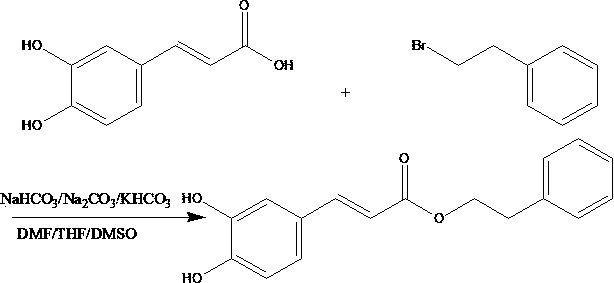
Preparation method for caffeic acid phenethyl ester
A technology of phenethyl caffeate and caffeic acid, which is applied in the field of preparation of phenethyl caffeate, can solve the problems of expensive materials, large environmental pollution, and many extraction steps, and achieve mild reaction conditions, small environmental pollution, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take 10g of caffeic acid, stir and dissolve it in 100mL of DMF and pour it into a round-bottomed flask, then add 30g of potassium bicarbonate as a catalyst, then add 9mL of β-phenylbromoethane, heat and stir under reflux at 80°C for 5 hours. Concentrate the solution under reduced pressure to saturation, pour out the concentrated solution and let it cool for crystallization for 6 hours, then filter out the crystals, rinse with 20mL of dichloromethane below 10°C, recover the lotion, and then rinse with 50mL of deionized water at 30°C Drying after washing to obtain the crude product of phenethyl caffeate;
[0019] Take 5g of the above crude phenethyl caffeate, add it to 30mL of toluene, heat it to 75°C and melt it, then pour it out while it is hot and let it cool for crystallization for 6 hours, then filter out the crystals and dry them to obtain the pure phenethyl caffeate. Yield≧88.6%, purity≧97.9% (HPLC).
Embodiment 2
[0021] Take 10g of caffeic acid, stir and dissolve it in 100mLTHF and pour it into a round-bottomed flask, then add 30g of potassium bicarbonate as a catalyst, then add 9mL of β-phenylbromoethane, heat and stir at 65°C for 5 hours. Concentrate the solution under reduced pressure to saturation, pour out the concentrated solution and let it cool for crystallization for 6 hours, then filter out the crystals, rinse with 20mL of dichloromethane below 10°C, recover the lotion, and then rinse with 50mL of deionized water at 30°C Drying after washing to obtain the crude product of phenethyl caffeate;
[0022] Take 5g of the above crude phenethyl caffeate, add it to 30mL of toluene, heat it to 75°C and melt it, then pour it out while it is hot and let it cool for crystallization for 6 hours, then filter out the crystals and dry them to obtain the pure phenethyl caffeate. Yield≧88.6%, purity≧97.9% (HPLC).
Embodiment 3
[0024] Take 10g of caffeic acid, stir and dissolve it in 100mLTHF and pour it into a round-bottomed flask, then add 30g of potassium bicarbonate as a catalyst, then add 9mL of β-phenylbromoethane, heat and stir at 65°C for 5 hours. Concentrate the solution under reduced pressure to saturation, pour out the concentrated solution and let it cool for crystallization for 6 hours, then filter out the crystals, rinse with 20mL of dichloromethane below 10°C, recover the lotion, and then rinse with 50mL of deionized water at 30°C Drying after washing to obtain the crude product of phenethyl caffeate;
[0025] Take 5g of the above crude product of phenethyl caffeate, add it to 30mL of cyclohexane, heat to 75°C and melt it, pour it out while it is hot and let it cool for crystallization for 6 hours, then filter out the crystals and dry them to obtain pure phenethyl caffeate. product, yield≧88.6%, purity≧97.9% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com