Application and preparation method of berberine compound
A compound and application technology, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problem of no quaternary ammonium berberine analgesic activity and the like, and achieve the effects of abundant synthetic raw materials, long-term analgesic effect and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Preparation of compound 2:
[0043] Dissolve 5 g of berberine hydrochloride in 400 mL of methanol, add 5 g of anhydrous potassium carbonate, dropwise add 6 ml of 5% sodium hydroxide solution (containing 0.4 g of sodium borohydride), stir and react at room temperature for one hour, filter out the precipitate, and use 30 % ethanol, washed with 80% ethanol, and recrystallized in methanol to obtain dihydroberberine (3.5 g, 76%). Dissolve dihydroberberine in 100mL of 80% ethanol and glacial acetic acid mixture, add 40ml of formaldehyde solution, reflux reaction at 85-95°C for 4-5 hours, remove the organic solvent by rotary evaporation, add an appropriate amount of concentrated hydrochloric acid to the residue, and leave at room temperature Under stirring for one hour, the solid was filtered out, washed with a small amount of water and methanol, and recrystallized in methanol to obtain compound 2 (3.6g, 90%)
[0044] Compound 2, C 21 h 20 NO 4 Cl, MW: 385.5 yellow crystal...
Embodiment 1
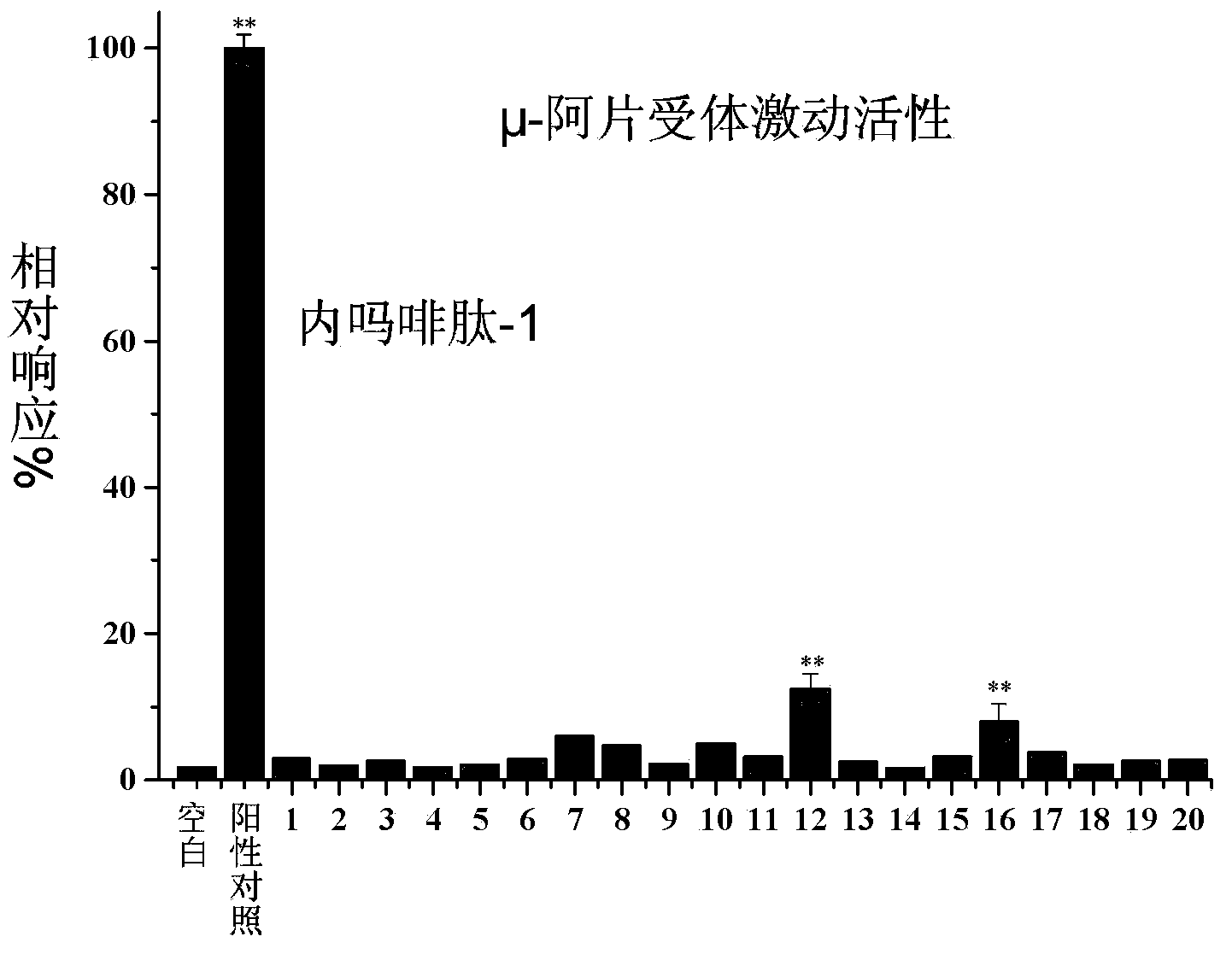
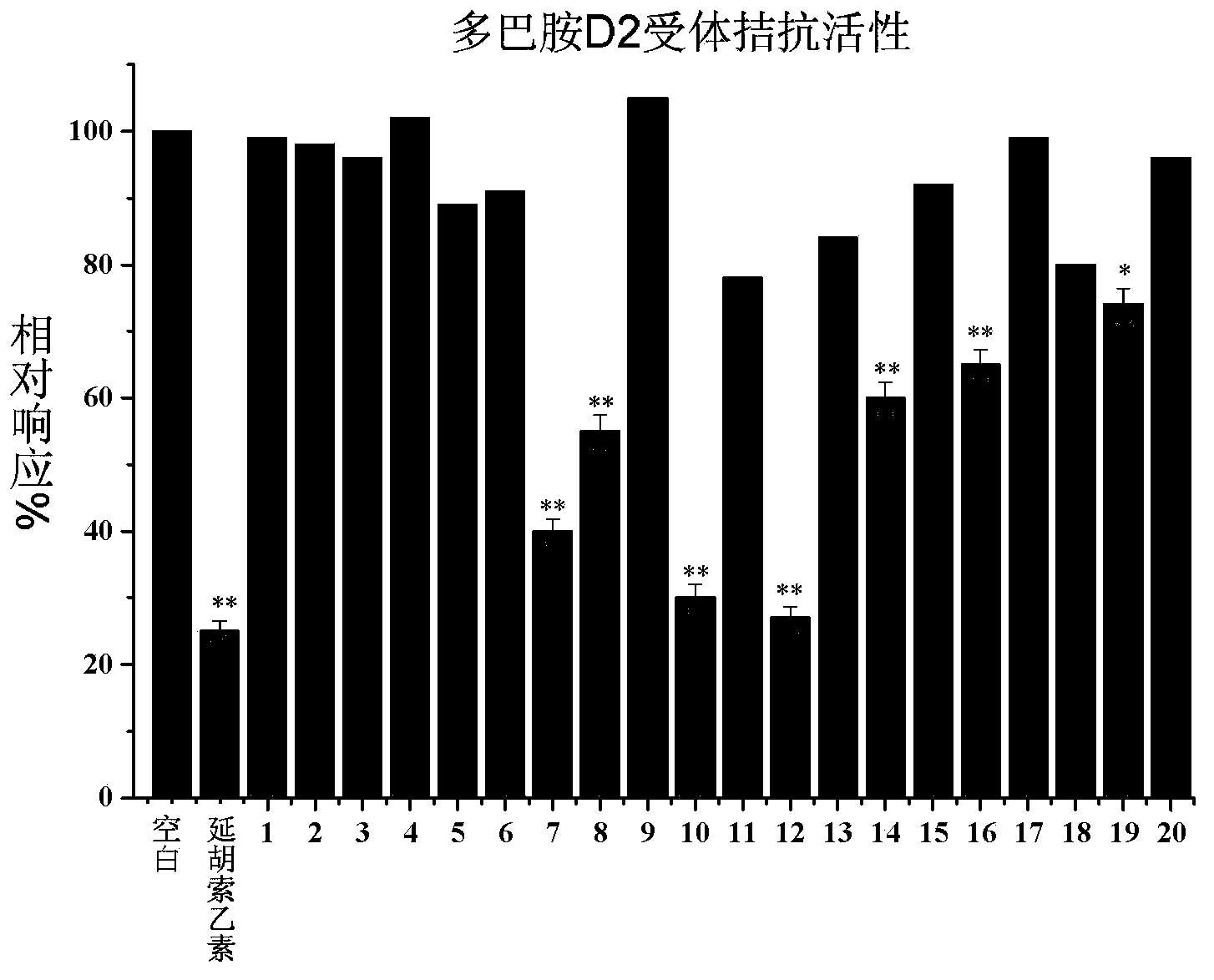
[0119] Activity Example 1: Using a cell receptor model, the effects of some compounds of the present invention on analgesia-related receptors were preliminarily evaluated in vitro.
[0120] Experimental protocol: The cells expressing mu opioid receptors used in this study were stably transfected human embryonic kidney 293T cells (HEK293T); the dopamine receptors used were transiently transfected HEK293T cells. Part of the berberine derivatives obtained in the present invention were added to cells expressing the corresponding receptors, and FLIPR (Fluorometric Imaging Plate Reader; Molecular Devices Corp) was used for functional screening and identification. Specifically: inoculate 80,000 cells per well in a poly-D-lysine-coated 96-well cell culture plate, remove the medium after 24 hours, and add 100 μl of fluorescent dye solution (2 μM Fluo-4AM first dissolves in each well) In pluronic acid, then dissolved in a buffer consisting of 1 times Hank's buffer and 20mM HEPES, pH 7.4...
Embodiment 2
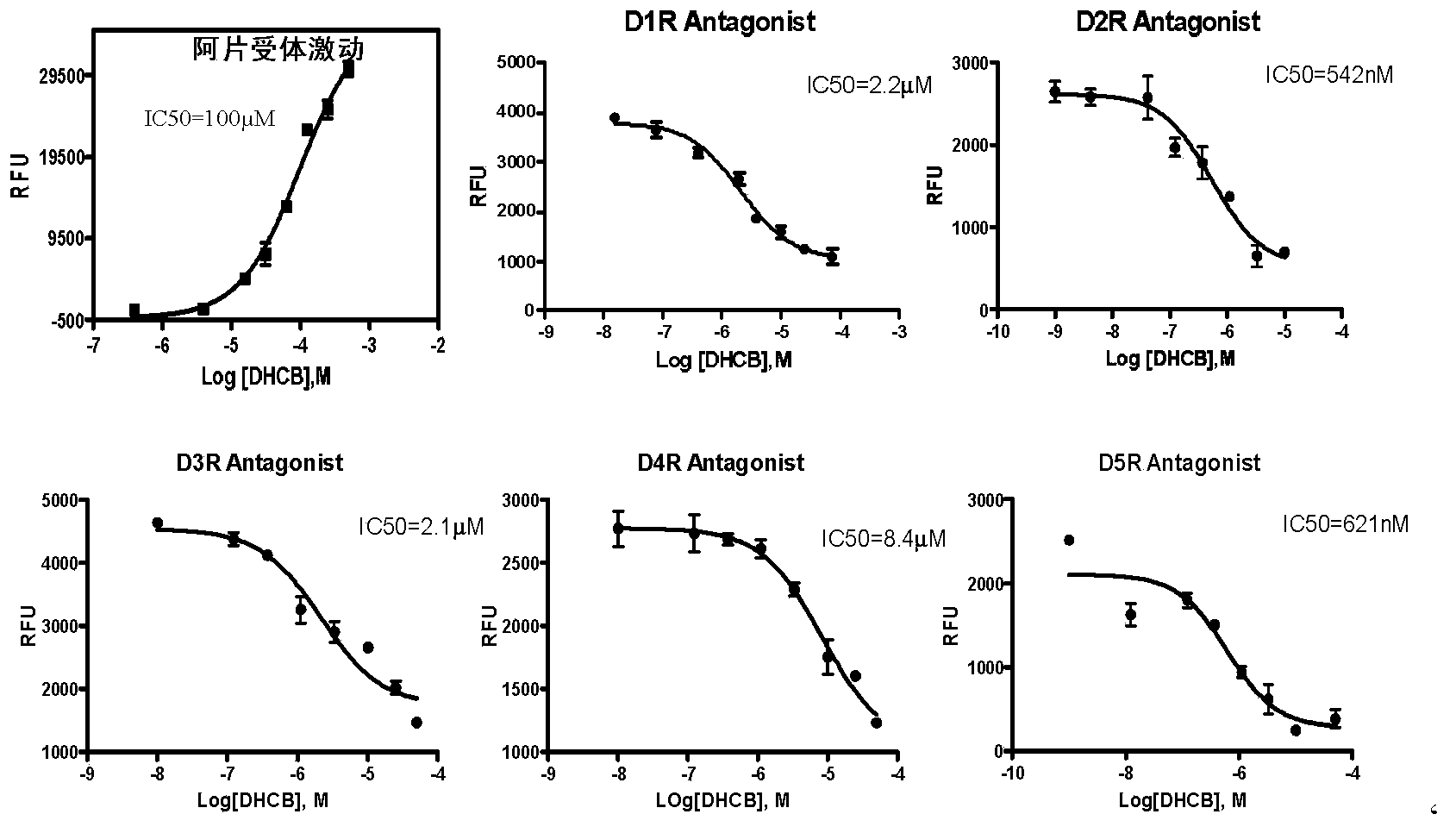
[0122] Activity Example 2: Comparison of dose-effect curves of dehydrocorydine (DHCB) to different receptor activities
[0123] The experimental scheme is the same as that in Example 1, except that when the test substance is added, the test substance is first diluted to different concentrations with a buffer to detect the response at different doses. In this way, the effects of DHCB on μ-opioid receptors and 5 kinds of dopamine receptors were detected, and the results are as follows: image 3 and shown in Table 1. Experiments have shown that DHCB is not only a weak agonist of μ-opioid receptors, but also an antagonist of various dopamine receptors (D1, D2, D3, D4, D5), which are not only related to pain, but also to Schizophrenia, depression and other mental diseases are related, suggesting that the compound also has certain therapeutic application value in this respect. In addition, the weak synergy of μ-opioid receptors can also enhance the analgesic effect of DHCB.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com