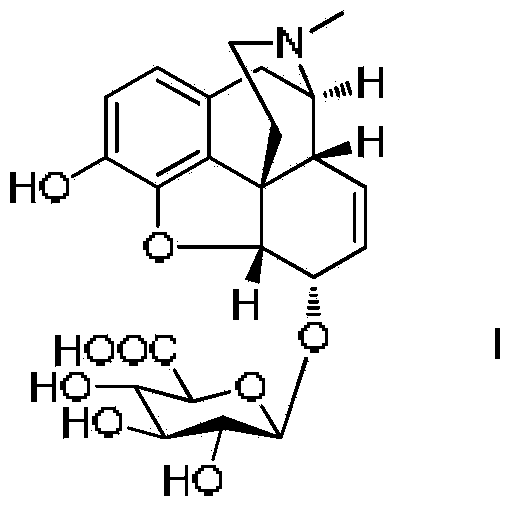
Synthesis method and intermediate compound of morphine-6-Beta-D-glucuronide
A technology of glucuronide and synthesis method, applied in the field of medicinal chemistry synthesis, can solve the problems of reduced yield, low yield, low feasibility of amplification and industrialization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
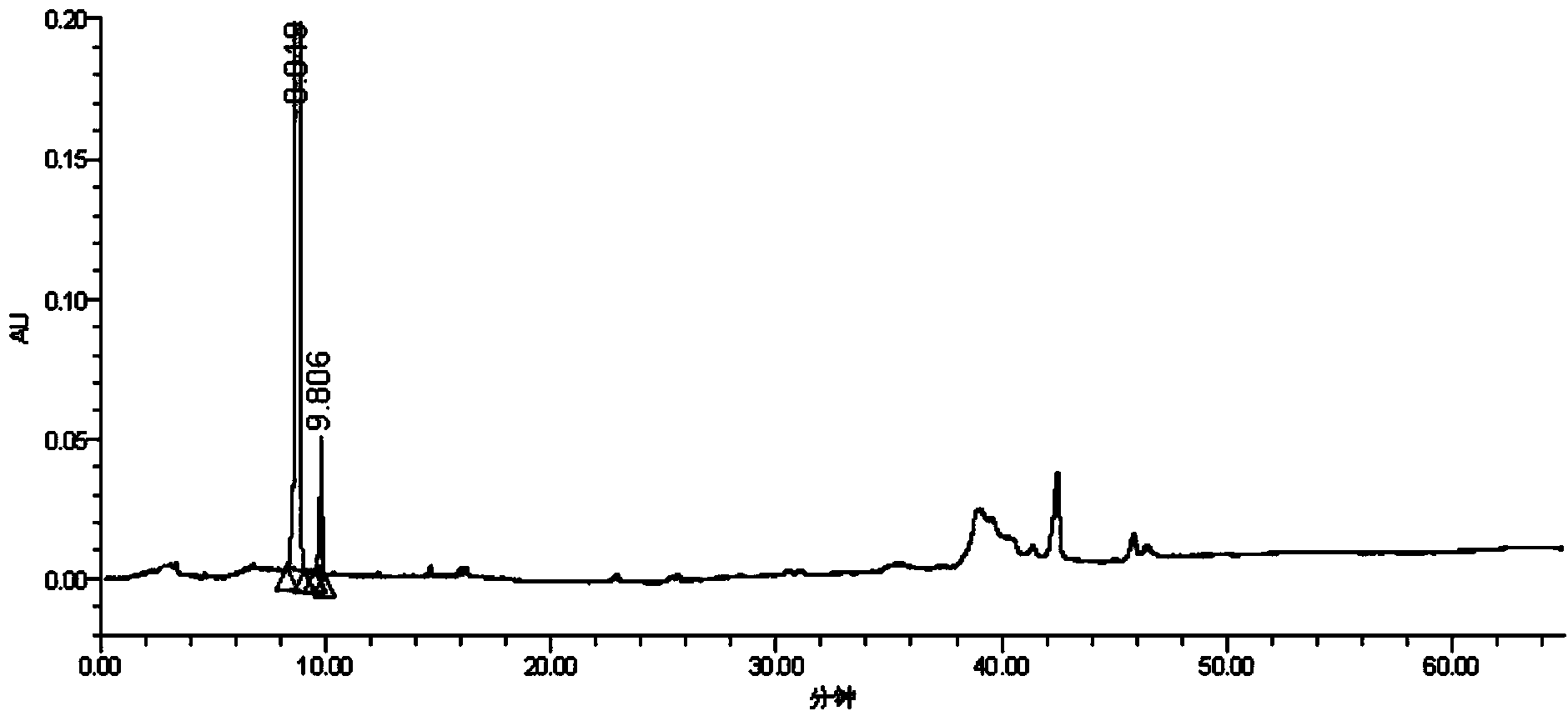
Image
Examples
Embodiment 1
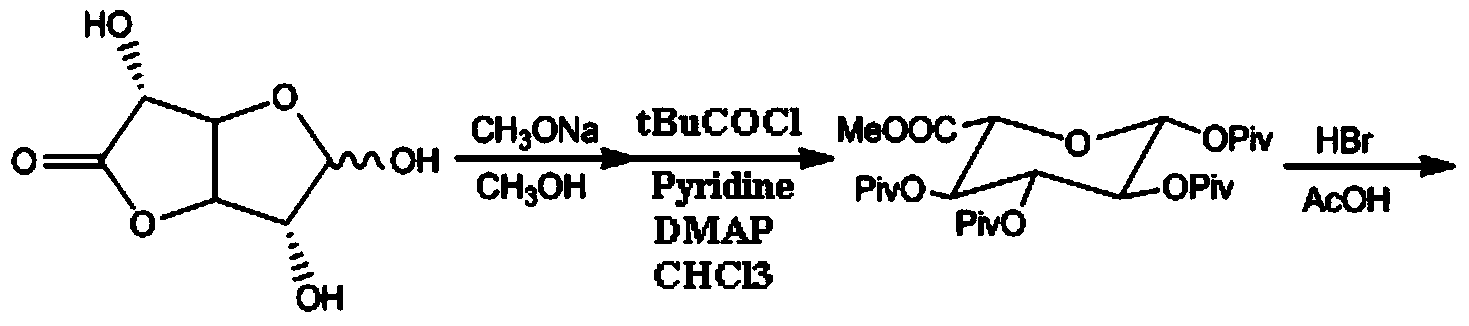
[0065] (1) Synthesis of Intermediate III (P1 and P2 are different protecting groups)
[0066] Dissolve 20 g of the compound shown in formula III (both P1 and P2 are isobutyryl, R is methyl) in acetic acid, add 10 g of trifluoroacetic anhydride, add 0.1 ml of boron trifluoride, react at 30-40 ° C, pour into ice In water, filtered, and the filter cake was dried under reduced pressure at 45-50°C to obtain (P1 is isobutyryl, P2 is trifluoroacetyl, R is methyl) 20.5 g of the compound represented by formula III, with a yield of 97.6%;
[0067] 1 H-NMR: (300MHz, CDCl 3 )δ=5.75(d, 1H), 5.11~5.16(m, 3H), 4.17(d, 1H), 3.74(s, 3H), 2.02(s, 9H).
[0068] (2) Synthesis of Intermediate IV
[0069] Dissolve 10 g of acetylmorphine (compound represented by formula II) and 20 g of compound represented by formula III (P1 is isobutyryl, P2 is trifluoroacetyl, R is methyl) in methyl tert-butyl ether, 10-25 ° C Add 5g of zinc bromide, react until complete at 55-60°C, filter, extract with dichlo...
Embodiment 2
[0079] 1) Synthesis of intermediate III (P1 and P2 are different protecting groups)
[0080] Dissolve 20 g of the compound shown in formula III (both P1 and P2 are acetyl, R is methyl) in benzoic acid, add 12 g of trichloromethanesulfonic anhydride, add 0.05 ml of boron trifluoride, react at 30-40 ° C, pour into Filter in ice water, and dry the filter cake under reduced pressure at 50-60°C to obtain 21.5 g of the compound represented by formula III (P1 is acetyl, P2 is trifluoromethanesulfonyl, R is methyl) with a yield of 97.5%;
[0081] 1 H-NMR: (300MHz, CDCl 3 )δ=6.42(d, 1H), 5.24~5.79(m, 3H), 4.44(d, 1H), 3.73(s, 3H), 2.46~2.54(m, 3H), 1.12(d, 18H).
[0082] (2) Synthesis of Intermediate IV
[0083] Dissolve 10 g of acetylmorphine (compound represented by formula II) and 18 g of compound represented by formula III (P1 is acetyl, P2 is trifluoromethanesulfonyl, R methyl) in dichloromethane, and add trifluoride Boron ether solution 15ml, 35 ~ 40 ° C until the reaction is...
Embodiment 3
[0088] (1) Synthesis of Intermediate III (P1 and P2 are different protecting groups)
[0089] Dissolve 20 g of the compound shown in formula III (both P1 and P2 are isopropionyl, R is ethyl) in benzoic acid, add 9 g of trifluoromethanesulfonic anhydride, add 3 g of zinc chloride, react at 70-80 ° C, pour into ice In water, filtered, and the filter cake was dried under reduced pressure at 50-60°C to obtain 20 g of the compound represented by formula III (P1 is isopropionyl, P2 is trifluoromethanesulfonyl, R is ethyl) with a yield of 96.5%;
[0090] (2) Synthesis of Intermediate IV
[0091] Dissolve 10 g of acetylmorphine (compound represented by formula II) and 16 g of compound represented by formula III (P1 is isopropionyl, P2 is trifluoromethanesulfonyl, R is ethyl) in chloroform, and add chlorine at 10-25 ° C Aluminum 12g, reacted at 40-45°C until complete, filtered, the filtrate was extracted with ethyl acetate, washed with water and saturated brine, concentrated to drynes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com