Method for producing recombinant human basic fibroblast growth factor from paddy rice seeds
A fibroblast and growth factor technology, applied in the field of genetic engineering, can solve problems such as basic fibroblast growth factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] [Example 1] Construction of rice-specific expression recombinant human basic fibroblast growth factor vector and preparation of transgenic rice plants
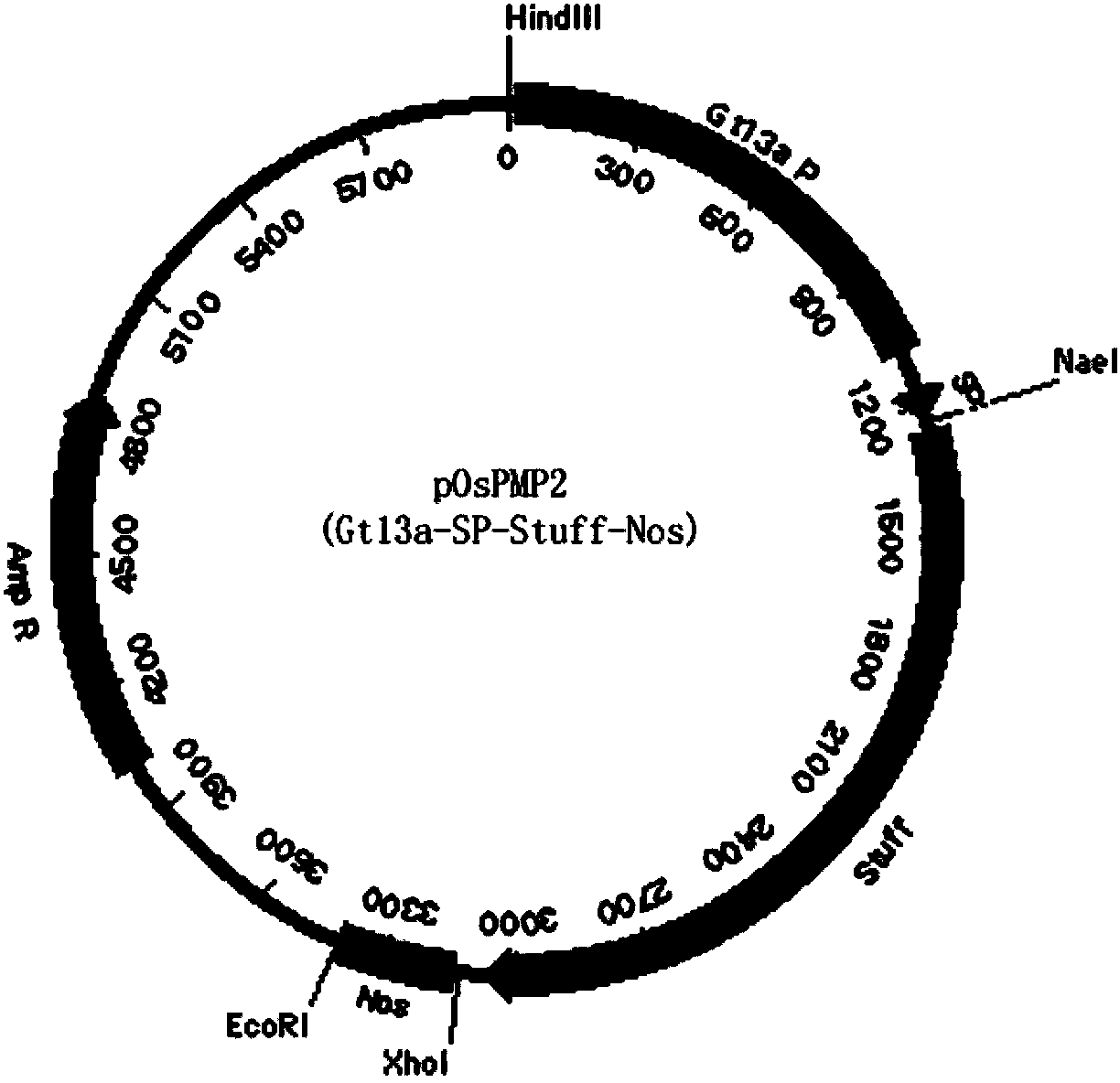
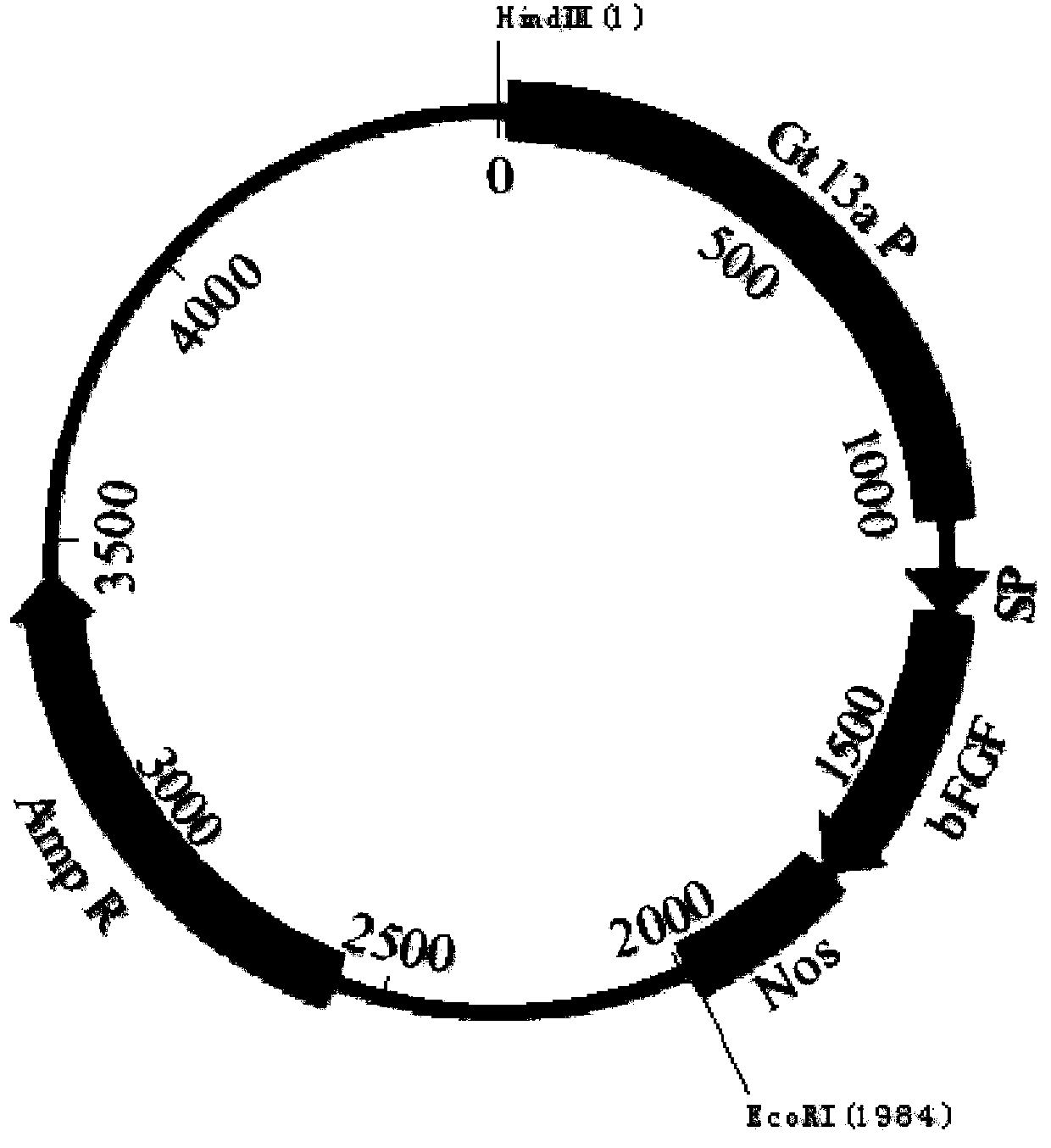
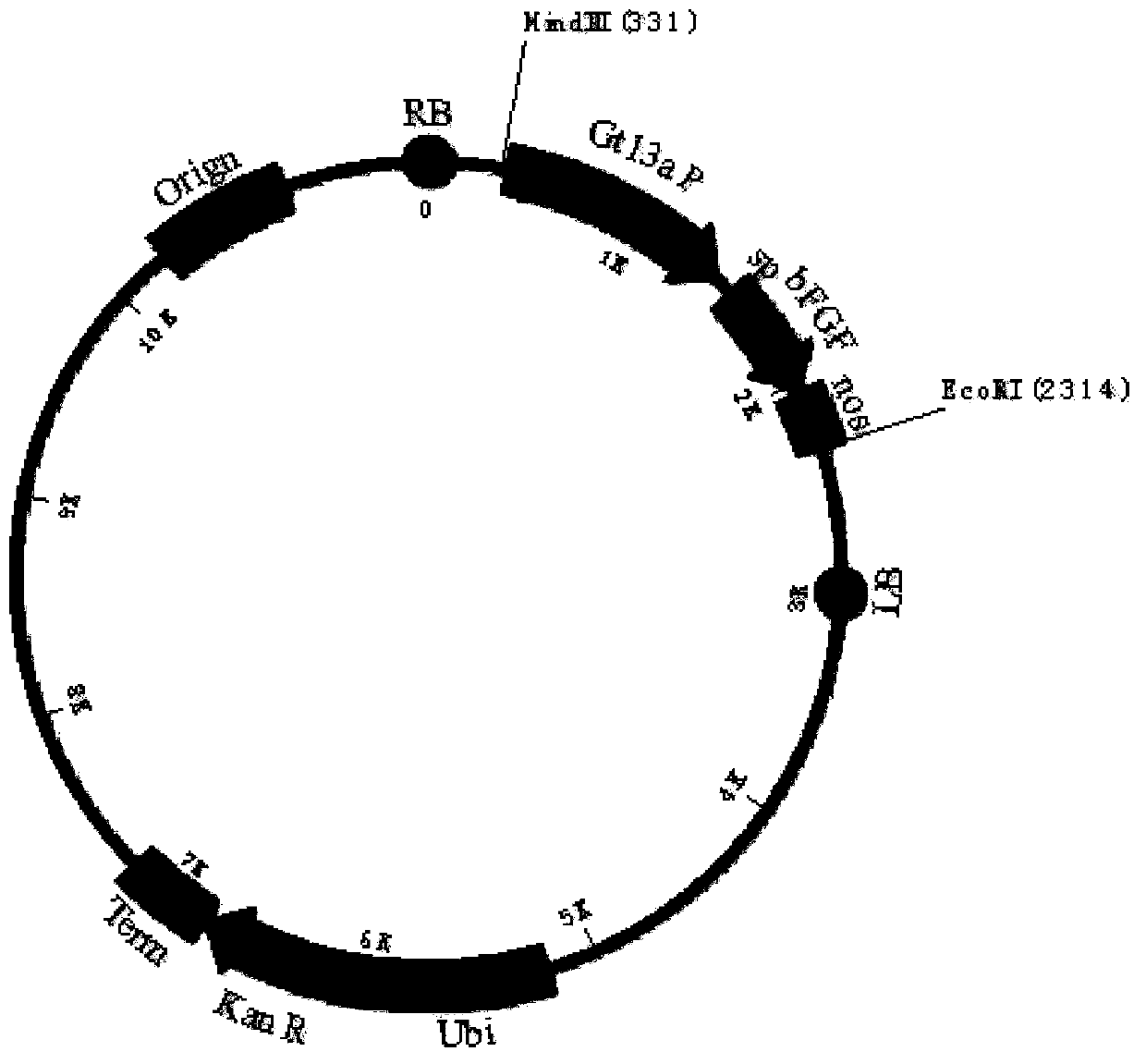
[0044]The human bFGF gene (Genbank accession number: NM002006) was synthesized by Heron Blue Biotechnology Company and optimized 61.54% of the genetic codons in the bFGF gene and made 21.65% of the nucleotides in the human bFGF gene Changes occur, and the optimized human bFGF gene sequence is shown in SEQ ID NO.1, and the amino acid sequence before and after codon optimization remains unchanged. In this embodiment, the rice-specific promoter Gt13a and its signal peptide are used to mediate the expression of recombinant human bFGF gene in rice endosperm cells, such as figure 1 The indicated plasmid pOsPMP2 was used to construct the rice endosperm-specific expression cassette. The synthetic codon-optimized human bFGF gene was digested with SchI and XhoI and cloned into pOsPMP2 to construct plasmid pOsPMP276, such as f...
Embodiment 2
[0045] [Example 2] Analysis and identification of OsrbFGF
[0046] In this example, Western hybridization was used to detect whether bFGF expressed in the obtained transgenic rice (referred to as OsrbFGF in the present invention) accumulated in rice endosperm cells. Take 100 mg of T1 seeds of the transgenic rice plants identified as positive by PCR, and grind them at 4°C for 60 min with 1 ml of extraction buffer (50 mM PB, pH 7.5, 1 mM EDTA, 1 mM L-reduced glutathione, 250 mM NaCl). minutes, and then centrifuged at 10620xg for 10 minutes to obtain crude protein extract. 40 μl crude protein extract and 350 ng Ecoli-derived recombinant bFGF were separated by 15% SDS-PAGE, and the gel was transferred to a nitrocellulose membrane. Rabbit polyclonal antibodies against basic fibroblast growth factor (Abcam, UK) and alkaline phosphatase goat anti-rabbit IgG (ZSGB-BIO, China), with nitro blue tetrazolium chloride (NBT) and 5-bromo -4-Chloro-3-indolyl phosphate (BCIP, BIOSHARP, China...
Embodiment 3
[0048] [Example 3] Isolation and purification of OsrbFGF
[0049] Grind and extract transgenic rice seeds with extraction buffer (50 mM PB, pH 7.5, 1 mM EDTA, 1 mM L-reduced glutathione, 250 mM NaCl) for 1 hour at room temperature, and the obtained extracts were passed through 3 μm and 0.22 μm The positive pressure filter was filtered and clarified, and then the filtrate was added to a Heparin 6 Fast Flow column (GE Healthcare, www.gelifesciences.com ), and equilibrated with 50mM PB containing 250mM NaCl and 1mM L-reduced glutathione; two-step washing method was used to remove foreign proteins and increase the recovery rate of OsrbFGF, wherein washing buffer I was (50mM PB (pH 7.5), 600mM NaCl, 1mML-reduced glutathione), washing buffer II is (50mM PB (pH 7.5), 900mM NaCl, 1mML-reduced glutathione); after washing, bind to Heparin OsrbFGF on the 6Fast Flow column was eluted with elution buffer (10mM Tris-HCl (pH 7.5), 1.7mM NaCl, 1mML-reduced glutathione) to obtain high-purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com