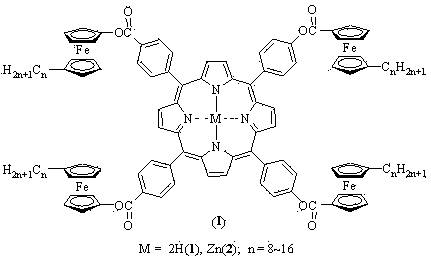
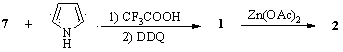Method for preparing one-class ferrocene modified porphyrin and metal porphyrin liquid crystal
A metalloporphyrin, ferrocene technology, applied in metallocene, chemical instruments and methods, organic chemistry and other directions, can solve problems such as no research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Series of compounds 3 Preparation of:
[0028] In a 250 mL three-neck flask equipped with a reflux condenser, add aluminum trichloride (3.5 g, 0.026 mol) and 30 mL of dichloromethane, and add long-chain n-alkyl acid chloride (0.015 mol) dropwise in an ice-water bath for 15 min. Dissolve the solution in 10 mL of dichloromethane, after dropping, continue to stir for 20 min. A solution of methyl ferrocenecarboxylate (3.0 g, 0.012 mol) dissolved in 20 mL of dichloromethane was dropped into the above system over 20 min under an ice-water bath, and the reaction was continued for 2 h after the addition was complete. Pour the reaction mixture into ice water to decompose, separate the organic phase, wash with 5% sodium carbonate solution and water successively, dry over anhydrous magnesium sulfate, and go through silica gel column chromatography with benzene as the eluent. The product is a brownish-red solid. The rate is 88%.
Embodiment 2
[0030] Series of compounds 4 Preparation of:
[0031] In a 250 mL three-neck flask, add zinc powder (13 g, 0.2 mol), wash with 4% dilute hydrochloric acid three times, pour off the supernatant, and wash with water several times. Add mercuric chloride (1.2 g, 0.017 mol) to 1 mL of concentrated hydrochloric acid to dissolve it, then add 20 mL of water to make a solution, add this solution to the above-mentioned three-neck flask and stir for 20 min to make zinc-amalgam. Methyl 1'-long chain acylferrocenecarboxylate 3 (10 mmol) was dissolved in 22 mL of benzene and 5.0 mL of methanol, added to a three-necked flask containing zinc-amalgam, heated to reflux, and 7 mL of concentrated hydrochloric acid was added dropwise, and refluxed for 3 h. Cool, filter with suction, and wash the filter cake with benzene. The organic phase was washed successively with saturated brine, 5% sodium carbonate solution and water, dried over anhydrous sodium sulfate, filtered, and evaporated to remove ...
Embodiment 3
[0033] Series of compounds 5 Preparation of:
[0034] Add sodium hydroxide (1.2 g, 0.3 mol) and 4.5 mL of water into a 50 mL round bottom flask, stir to dissolve the sodium hydroxide. Then add 4.5 mL of 1'-long-chain n-alkylferrocene formic acid methyl ester 4(7.4 mmol) ethanol solution, heated in a boiling water bath, and monitored the reaction process by thin-layer chromatography. Benzene was used as the developer. After the reaction was completed, 20 mL of water was added to dilute, extracted with ether, and the aqueous phase was adjusted to pH = 1 with dilute hydrochloric acid to precipitate out , the precipitate was dissolved in ether, washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to obtain a reddish-brown solid with a yield of 89%.
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com