Multihole metal porphyrin organic covalent polymeric material and preparation method and application of material
A polymeric material and porous metal technology, applied in the field of a porous metalloporphyrin organic covalent polymeric material and its preparation, can solve the problems of poor selectivity, high requirements for industrial equipment, and low catalyst efficiency, and achieve low cost and selectivity. High performance and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
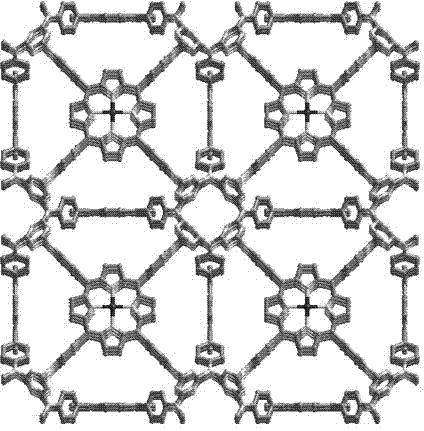
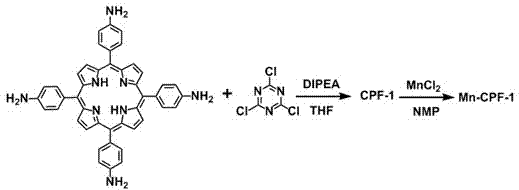
[0022] Such as figure 2 As shown, a preparation method of a porous metalloporphyrin organic covalent polymer material, TAPP (tetraaminophenylporphyrin) as the basic raw material, reacts with cyanuric chloride under mild conditions to polymerize into a high polymer, and uses This is the basic carrier, by using the porphyrin macrocycle to support the metal Mn III The ions modulate the catalytic performance of the polymer. Specific steps are as follows:
[0023] (1) Dissolve 0.1~5 mmol TAPP (tetraaminophenylporphyrin) and 0.1~5 mL diisopropylethylamine in 5~200 mL THF (tetrahydrofuran) solvent, add 5~500 mL dropwise to the above solvent mL of tetrahydrofuran solution of cyanuric chloride, wherein the content of cyanuric chloride is 0.03 ~ 13.5 mmol, react in ice bath for 8 hours, then raise the temperature to room temperature within 4 hours and react for 12 hours, then at 90 o C was reacted for 48 hours. Collect the precipitate obtained by the reaction by filtering and washi...
Embodiment 1
[0029] A porous metalloporphyrin organic covalent polymer material, the preparation method is as follows:
[0030] Dissolve 0.75 mmol TAPP and 1 mL diisopropylethylamine in 40 mL THF solvent, add 40 mL cyanuric chloride solution in tetrahydrofuran dropwise to the above solvent, wherein the content of cyanuric chloride is 2 mmol, and put it in an ice bath Reacted for 8 hours, then the temperature was slowly raised to room temperature in 4 hours and reacted for 12 hours, then at 90 o C was reacted for 48 hours. The resulting precipitate from the reaction was collected by filtration and washed successively with tetrahydrofuran, chloroform and water to obtain the porphyrin polymer CPF-1, which was dried; the metal Mn 2+ Ions and porphyrin polymer CPF-1 were fed into 50mL NMP according to the molar ratio of 5:1, at 100 oC After 12 hours of reaction, 200 mL of methanol was poured into, and the metal-porphyrin polymer material Mn-CPF-1 was precipitated, and washed with 5% dilute h...
Embodiment 2
[0032] A porous metalloporphyrin organic covalent polymer material, the preparation method is as follows:
[0033] Dissolve 0.1 mmol TAPP and 0.1 mL diisopropylethylamine in 5 mL THF solvent, add 5 mL cyanuric chloride solution in tetrahydrofuran dropwise to the above solvent, wherein the content of cyanuric chloride is 0.03 mmol, and place in an ice bath Reacted for 8 hours, then the temperature was slowly raised to room temperature in 4 hours and reacted for 12 hours, then at 90 oC was reacted for 48 hours. The resulting precipitate from the reaction was collected by filtration and washed successively with tetrahydrofuran, chloroform and water to obtain the porphyrin polymer CPF-1, which was dried; the metal Mn 2+ Ions and porphyrin polymer CPF-1 were fed into 50mL NMP according to the molar ratio of 5:1, at 100 oC After 12 hours of reaction, 200 mL of methanol was poured into, and the metal-porphyrin polymer material Mn-CPF-1 was precipitated, and washed with 5% dilute h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com