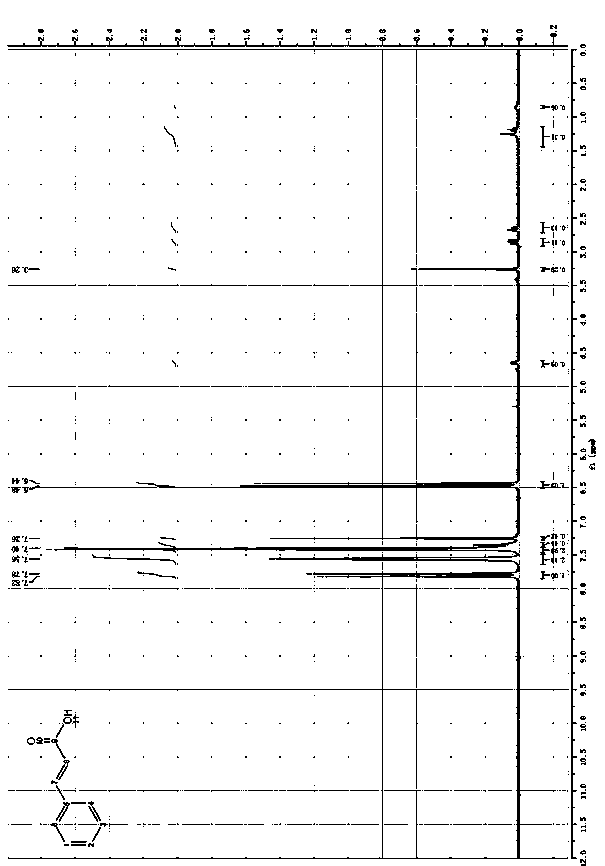
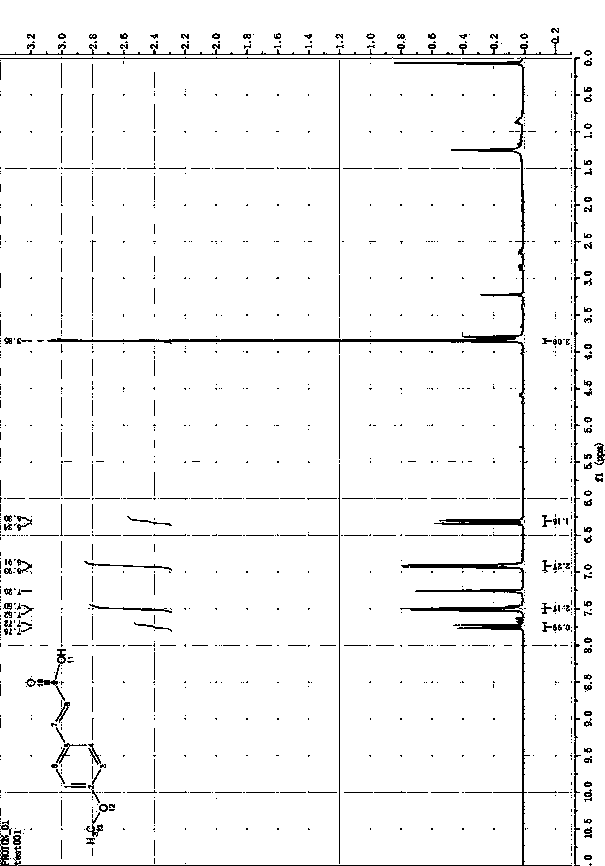
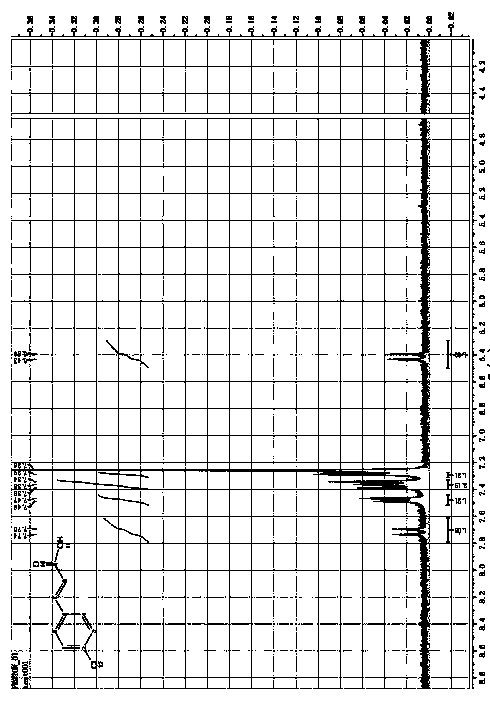
Preparation method of cinnamic acid or derivatives thereof
A technology of derivatives and cinnamic acid, which is applied in the field of preparation of cinnamic acid or its derivatives, can solve problems such as adverse effects on the nervous system and respiratory tract, difficulties in industrial scale-up production, and environmental pollution from emissions, so as to avoid the use of toxic catalysts and overcome It is difficult to recover raw materials and avoid the effect of high temperature reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, a kind of preparation method of cinnamic acid or derivative thereof, its steps are as follows:
[0036] (1) Esterification reaction: Add triethyl phosphonoacetate in dichloromethane solution into the reaction vessel, slowly add sodium hydride under stirring at -5°C, raise the temperature to 20°C, quickly add the raw material aldehyde, and react at room temperature for 2~ After 6 hours, add a saturated aqueous solution of ammonium chloride and stir, the organic phase is washed successively with saturated sodium bicarbonate solution and saturated sodium chloride solution, the solvent is removed under reduced pressure, and purified by column chromatography to obtain a light yellow oil;
[0037] In the esterification reaction, the ratio of the amount of raw materials is, aldehyde: triethyl phosphonoacetate: sodium hydride=1:1.2:2.5;
[0038] Described aldehyde is selected from benzaldehyde, p-methoxybenzaldehyde, p-chlorobenzaldehyde, p-fluorobenzaldehyde, 2-t...
Embodiment 2
[0041] Embodiment 2, a kind of preparation method of cinnamic acid or derivative thereof, its steps are as follows:
[0042] (1) Esterification reaction: Add triethyl phosphonoacetate in dichloromethane solution into the reaction vessel, slowly add sodium hydride under stirring at 5°C, raise the temperature to 30°C, quickly add raw material aldehyde, and react at room temperature for 2-6 hour, add a saturated aqueous solution of ammonium chloride and stir, the organic phase is washed with saturated sodium bicarbonate solution and saturated sodium chloride solution successively, the solvent is removed under reduced pressure, and purified by column chromatography to obtain a light yellow oil;
[0043] In the esterification reaction, the ratio of the amount of raw materials is, aldehyde: triethyl phosphonoacetate: sodium hydride=1: 1.8: 3.5;
[0044] Described aldehyde is selected from benzaldehyde, p-methoxybenzaldehyde, p-chlorobenzaldehyde, p-fluorobenzaldehyde, 2-thiophene fo...
Embodiment 3
[0047] Embodiment 3, a kind of preparation method of cinnamic acid or derivative thereof, its steps are as follows:
[0048](1) Esterification reaction: Add triethyl phosphonoacetate in dichloromethane solution into the reaction vessel, slowly add sodium hydride under stirring at 0°C, raise the temperature to room temperature, quickly add the raw material aldehyde, and react at room temperature for 2 to 6 hours , adding a saturated aqueous solution of ammonium chloride and stirring, the organic phase was washed with saturated sodium bicarbonate solution and saturated sodium chloride solution successively, the solvent was removed under reduced pressure, and purified by column chromatography to obtain a light yellow oil;
[0049] In the esterification reaction, the ratio of the amount of raw materials is, aldehyde: triethyl phosphonoacetate: sodium hydride=1:1.5:3;
[0050] Described aldehyde is selected from benzaldehyde, p-methoxybenzaldehyde, p-chlorobenzaldehyde, p-fluoroben...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com