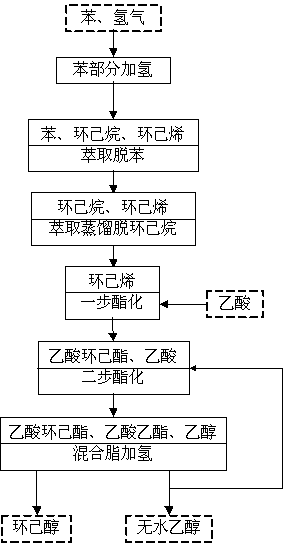
Method for co-production of cyclohexanol and absolute ethyl alcohol
A technology of anhydrous ethanol and cyclohexanol, applied in chemical instruments and methods, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of acetic acid vapor being corrosive to equipment and the mixture cannot be separated , high energy consumption in the distillation process, etc., to achieve the effect of realizing full utilization of materials, reducing equipment requirements and production energy consumption, and improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Add 30 L of benzene, 500 g of Ru-ZnSO to the benzene hydrogenation reactor 4 -Zn(OH) 2 -ZrO 2 / SiO 2 (where Ru, ZnSO 4 , Zn(OH) 2 and ZrO 2 The mass percent composition in the catalyst is respectively 5%, 1%, 1% and 2%) and HZSM-10 molecular sieve 300 g, stir after sealing, be heated to 150 DEG C, then charge into hydrogen in the reactor, make The partial pressure was 5 MPa, and the reaction was carried out for 0.5 hours. A small amount of reaction solution was taken out by the sampling valve and analyzed by gas chromatography. It can be seen that the conversion rate of benzene was 55.3%, and the yield of cyclohexene was 35.2%. After the benzene hydrogenation reaction is completed, the oil phase in the kettle is dewatered to reduce the water content to below 15 ppm.
[0055] (2) Use DMAc as the extractant to extract and remove benzene from the above-mentioned oil phase after dehydration, the flow rate of DMAc is 0.18 kmol / h, and the volume ratio of DMAc to t...
Embodiment 2
[0061] (1) Add 30 L of benzene, 500 g of Ru-ZnSO to the benzene hydrogenation reactor 4 -Zn(OH) 2 -ZrO 2 / SiO 2 (where Ru, ZnSO 4 , Zn(OH) 2 and ZrO 2 The mass percent composition in the catalyst is respectively 5%, 1%, 1% and 2%) and HZSM-10 molecular sieve 300 g, stir after sealing, be heated to 150 DEG C, then charge into hydrogen in the reactor, make The partial pressure is 5 MPa, and the reaction time is 0.5 hours. A small amount of reaction solution was taken out by the sampling valve and analyzed by gas chromatography. It can be seen that the conversion rate of benzene was 55.3%, and the yield of cyclohexene was 35.2%. After the benzene hydrogenation reaction is completed, the oil phase in the kettle is dewatered to reduce the water content to below 15 ppm.
[0062] (2) Use DMAc as the extractant to extract and remove benzene from the above-mentioned oil phase after dehydration, the flow rate of DMAc is 0.18 kmol / h, and the volume ratio of DMAc to the oil phase ...
Embodiment 3
[0068] (1) Add 30 L of benzene, 500 g of Ru-ZnSO to the benzene hydrogenation reactor 4-Zn(OH) 2 -ZrO 2 / SiO 2 (where Ru, ZnSO 4 , Zn(OH) 2 and ZrO 2 The mass percent composition in the catalyst is respectively 5%, 1%, 1% and 2%) and HZSM-10 molecular sieve 300 g, stir after sealing, be heated to 150 DEG C, then charge into hydrogen in the reactor, make The partial pressure was 5 MPa, and the reaction was carried out for 0.5 hours. A small amount of reaction solution was taken out by the sampling valve and analyzed by gas chromatography. It can be seen that the conversion rate of benzene was 55.3%, and the yield of cyclohexene was 35.2%. After the benzene hydrogenation reaction is completed, the oil phase in the kettle is dewatered to reduce the water content to below 15 ppm.
[0069] (2) Use DMAC as the extractant to extract and remove benzene from the above-mentioned oil phase after the dehydration treatment, the DMAC flow rate is 0.18 kmol / h, the volume ratio of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com