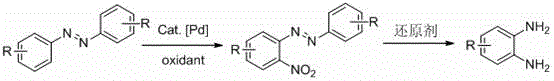
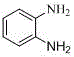
A kind of preparation method of o-phenylenediamine and its derivatives
A technology of o-phenylenediamine and its derivatives, which is applied in the field of synthesis of organic compounds, can solve the problems of difficult purification of by-products, complex process routes, harsh reaction conditions, etc., achieve simple routes, avoid waste acid pollution, and have low equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 54.6 mg (0.3 mmol) azobenzene, 6.7 mg (0.03 mmol) palladium acetate, 92.3 mg (0.6 mmol) silver nitrite, 162.2 mg (0.6 mmol) potassium persulfate, 3.5 mL DCE into a closed reaction vessel , the reaction mixture was stirred and reacted at 110° C. for 72 hours. After the reaction stopped, cool to room temperature, add 10 mL of dichloromethane to the reaction solution, remove the solvent after suction filtration under reduced pressure, and the residue was subjected to column chromatography [GF254 silica gel (100-200 mesh); eluent was V (petroleum ether) / V(ethyl acetate)=6 / 1] separation and purification to obtain reddish-brown solid 2-nitroazobenzene with a yield of 95%. 2-Nitroazobenzene (0.3 mmol), zinc powder (0.36 mmol), and formic acid (2 mL) were mixed and reacted in methanol solvent under nitrogen atmosphere for 24 hours at room temperature to obtain o-benzene with a yield of 88%. diamine.
[0026]
[0027] mp 102-104 ºC; IR (neat): n = 3352 (NH2) cm -1 ; 1H...
Embodiment 2
[0029] Add 54.6 mg (0.3 mmol) of azobenzene, 0.03 mmol of palladium chloride, 0.6 mmol of potassium nitrite, 0.6 mmol of ceric ammonium nitrate, 3.5 mL of DCE into a closed reaction vessel, and stir the reaction mixture at 110°C for 48 Hour. After the reaction stopped, cool to room temperature, add 10 mL of dichloromethane to the reaction solution, remove the solvent after suction filtration under reduced pressure, and the residue was subjected to column chromatography [GF254 silica gel (100-200 mesh); eluent was V (petroleum ether) / V(ethyl acetate)=6 / 1] separation and purification to obtain reddish-brown solid 2-nitroazobenzene with a yield of 70%. 2-Nitroazobenzene (0.3 mmol), zinc powder (0.45 mol), and formic acid (3 mL) were mixed and reacted in a methanol solvent under nitrogen at room temperature for 24 hours to obtain o-benzene with a yield of 80%. diamine.
[0030]
[0031] mp 102-104 ºC; IR (neat): n = 3352 (NH2) cm -1 ; 1H NMR (CDCl3, 500MHz): δ 6.76-6.72 (m, ...
Embodiment 3
[0033] Add 0.3 mmol of azobenzene, 0.03 mmol of diacetonitrile palladium dichloride, 0.6 mmol of sodium nitrite, 0.6 mmol of potassium persulfate, 3.5 mL of DCE into a closed reaction vessel, and stir the reaction mixture at 110°C for 72 hours . After the reaction stopped, cool to room temperature, add 10 mL of dichloromethane to the reaction solution, remove the solvent after suction filtration under reduced pressure, and the residue was subjected to column chromatography [GF254 silica gel (100-200 mesh); eluent was V (petroleum ether) / V(ethyl acetate)=6 / 1] separation and purification to obtain reddish-brown solid 2-nitroazobenzene with a yield of 56%. 2-Nitroazobenzene (0.3 mmol), zinc powder (0.36 mol), and formic acid (2 mL) were mixed and reacted in methanol solvent under nitrogen at room temperature for 24 hours to obtain o-benzene with a yield of 88%. diamine.
[0034]
[0035] mp 102-104 ºC; IR (neat): n = 3352 (NH2) cm -1 ; 1H NMR (CDCl3, 500MHz): δ 6.76-6.72 (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com